Talk:Microscopic identification of salts
Author: Hans-Jürgen Schwarz
back to Polarized light microscopy or Analysis of Salts
Abstract[edit]
This section describes the determination of salts using polarized light microscopy.
Method[edit]
The examination of salts can be carried out using several different methods, the present article focuses on two different approaches to the microscopic analysis of salts.
The first method is based on the examination of the salts, i.e., salt crystals as taken from the object. The second method analyses the salt as it re-crystallizes from an aqueous solution of the sample. The salt crystallization obtained from solution may vary from that present in the original sample, depending on the different kinds of salt preset as described below. Please see also Micro-chemical testing.
It is important to remember that in conventional chemical analysis the presence of the carbonate anion is not carried out routinely, so that its presence may be overlooked, although they are often found in building materials and may cause their deterioration.
Determination of single salts in aqueous extract[edit]
The sample to be examined (pure salt or material/salt mixture) is mixed with a few drops of distilled water, producing an aqueous salt solution with reference to Bläuer. A few drops of this solution are placed onto a microscope slide and observed under the polarizing microscope. It is important to observe the crystallization of the salts from the beginning, i.e., from the appearance of the first small crystals until the end of the crystallization process,because salts that crystallize later may cover up some of the initial salt crystals completely and therefore, if not identified at the beginning, their presence may become difficult, if not impossible, to see. The continuous documentation of the process is therefore important for an accurate analysis. Only then, the right conclusions can be drawn.
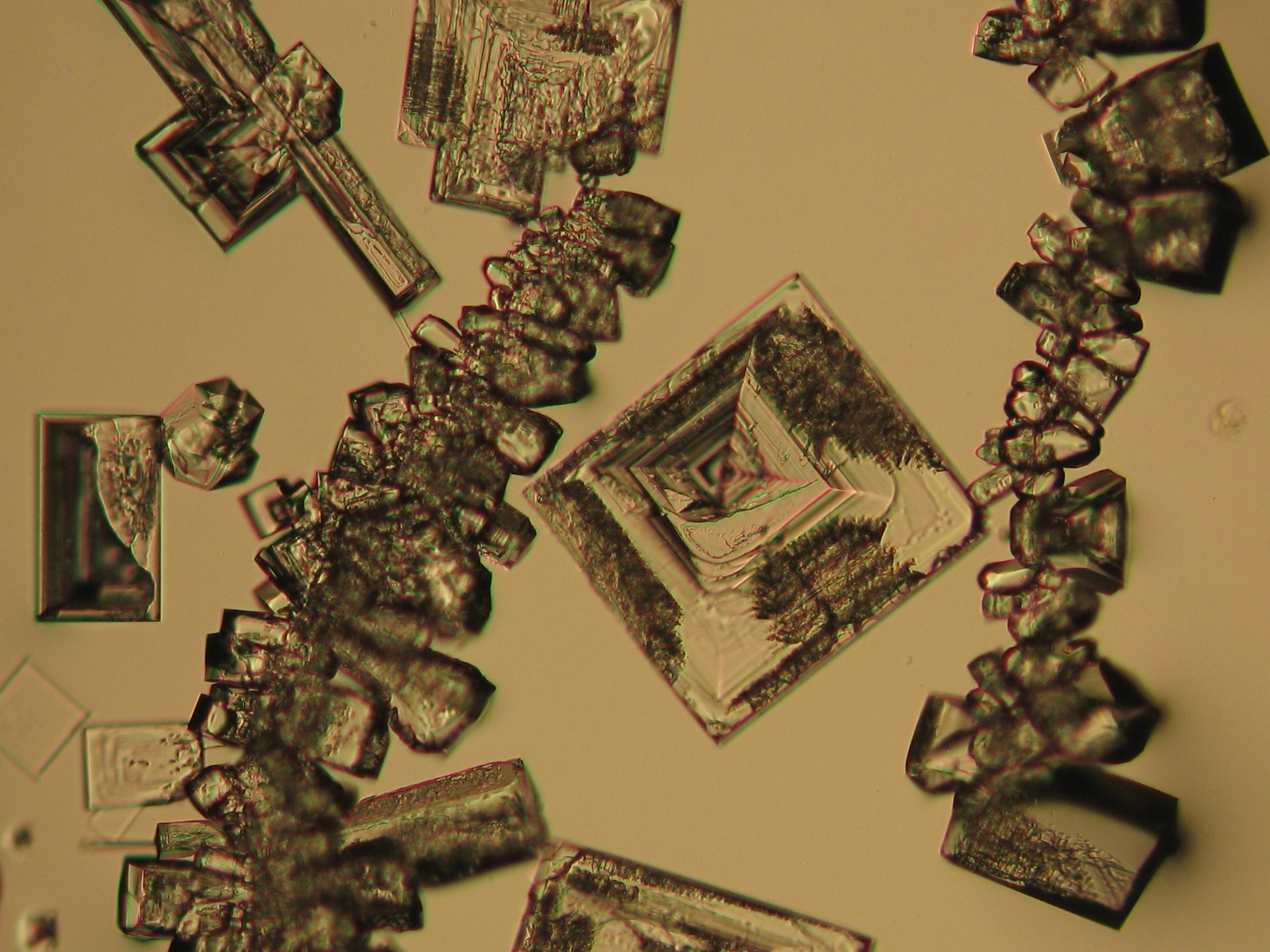
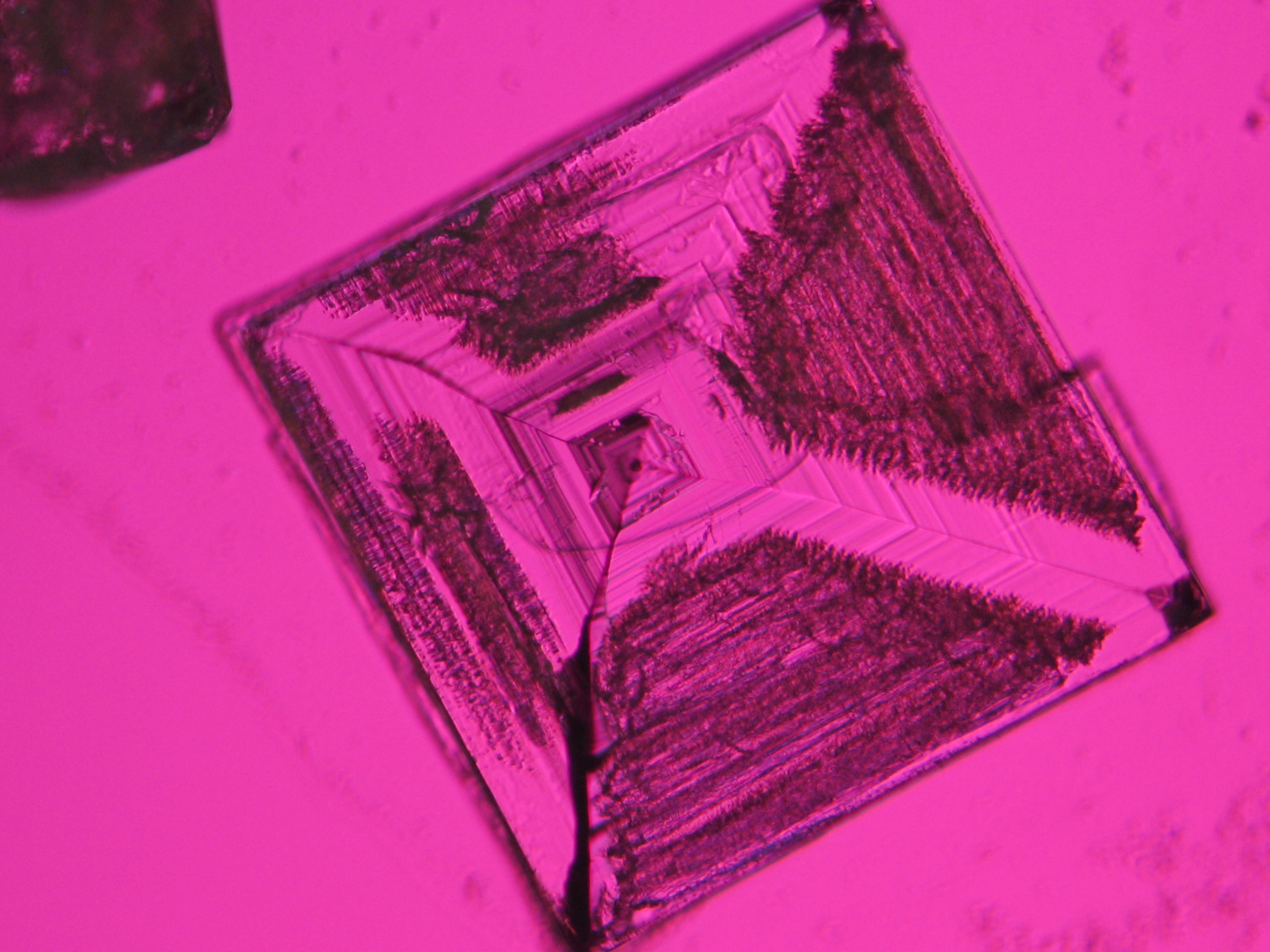
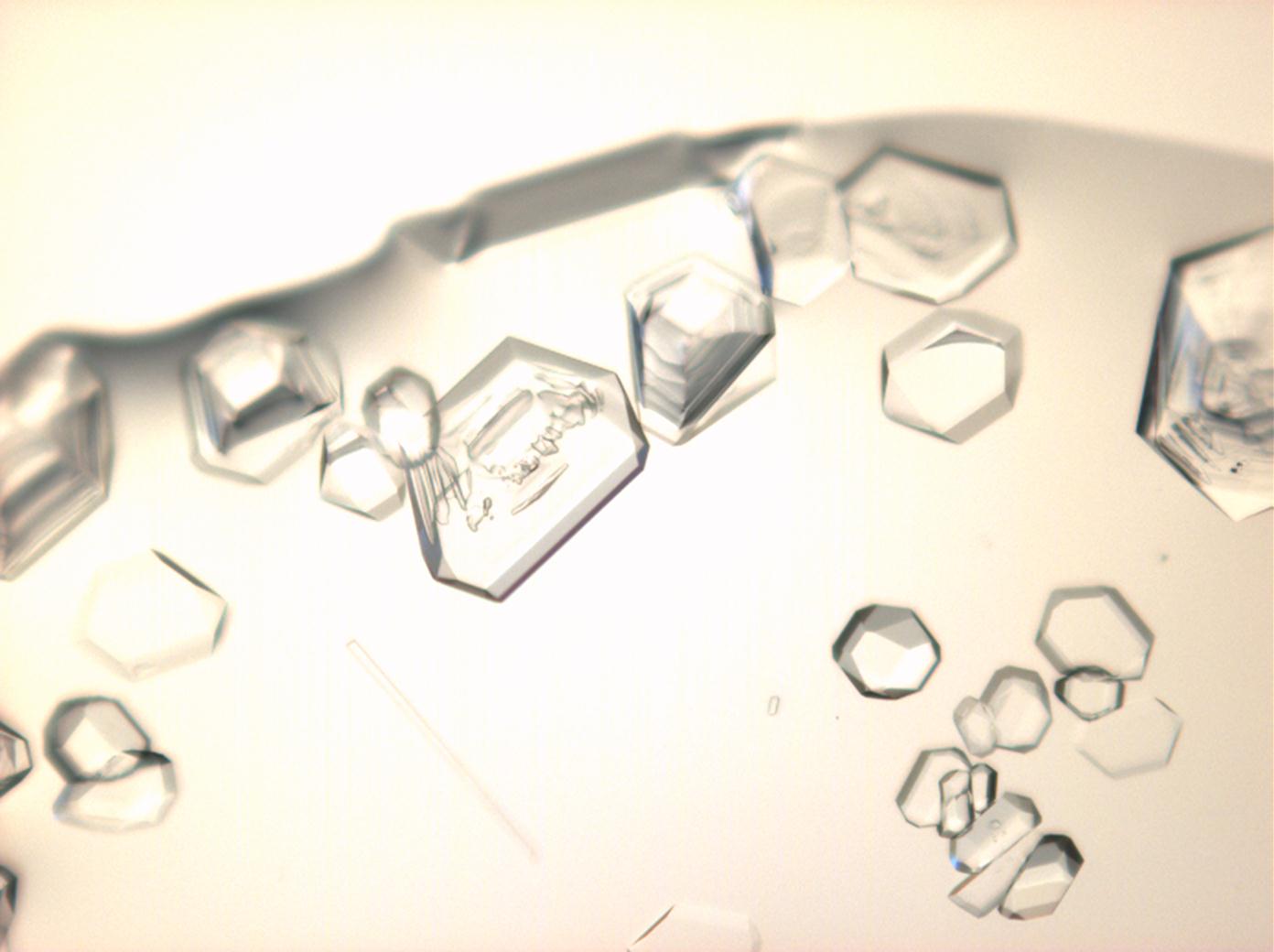
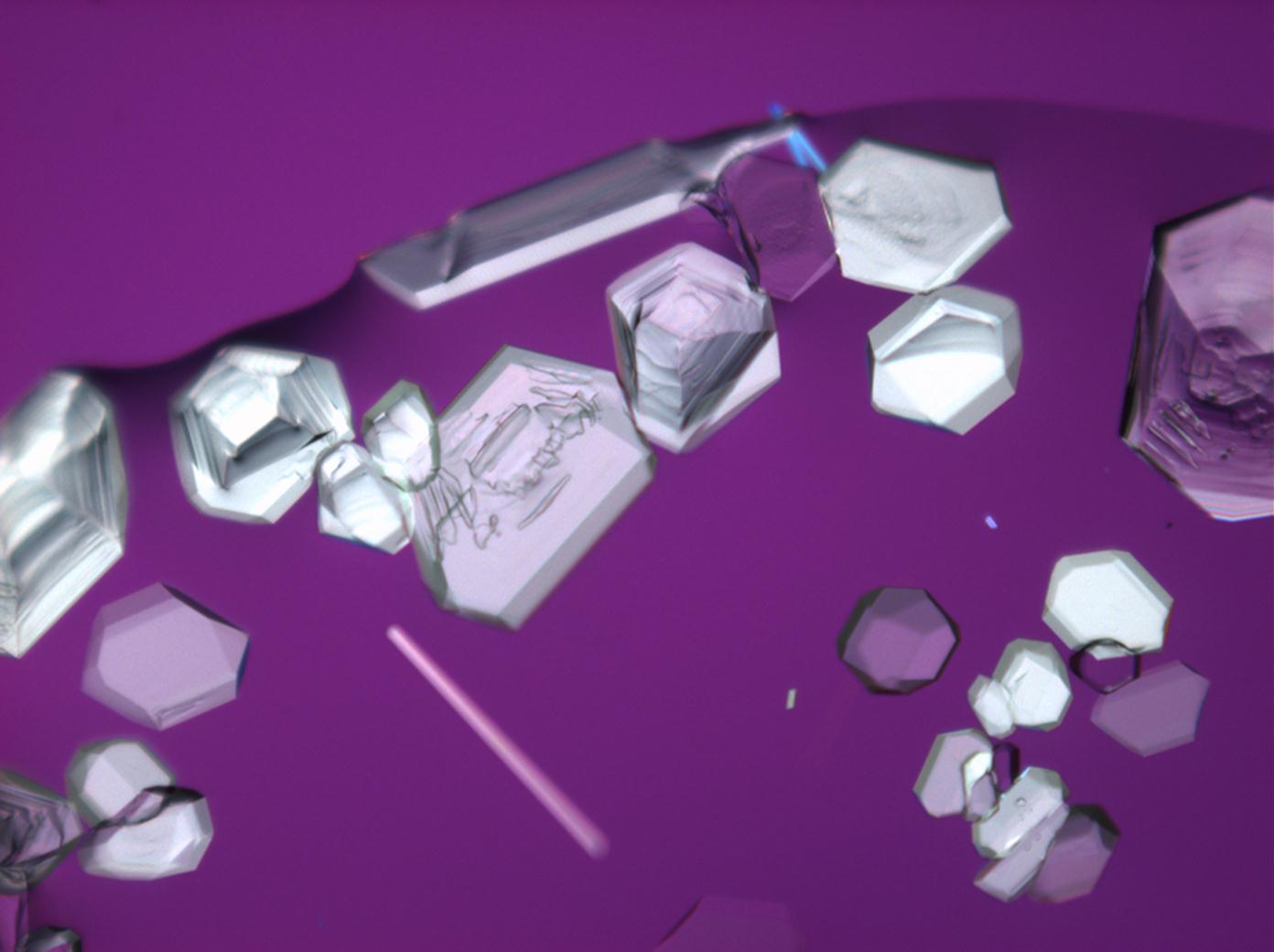
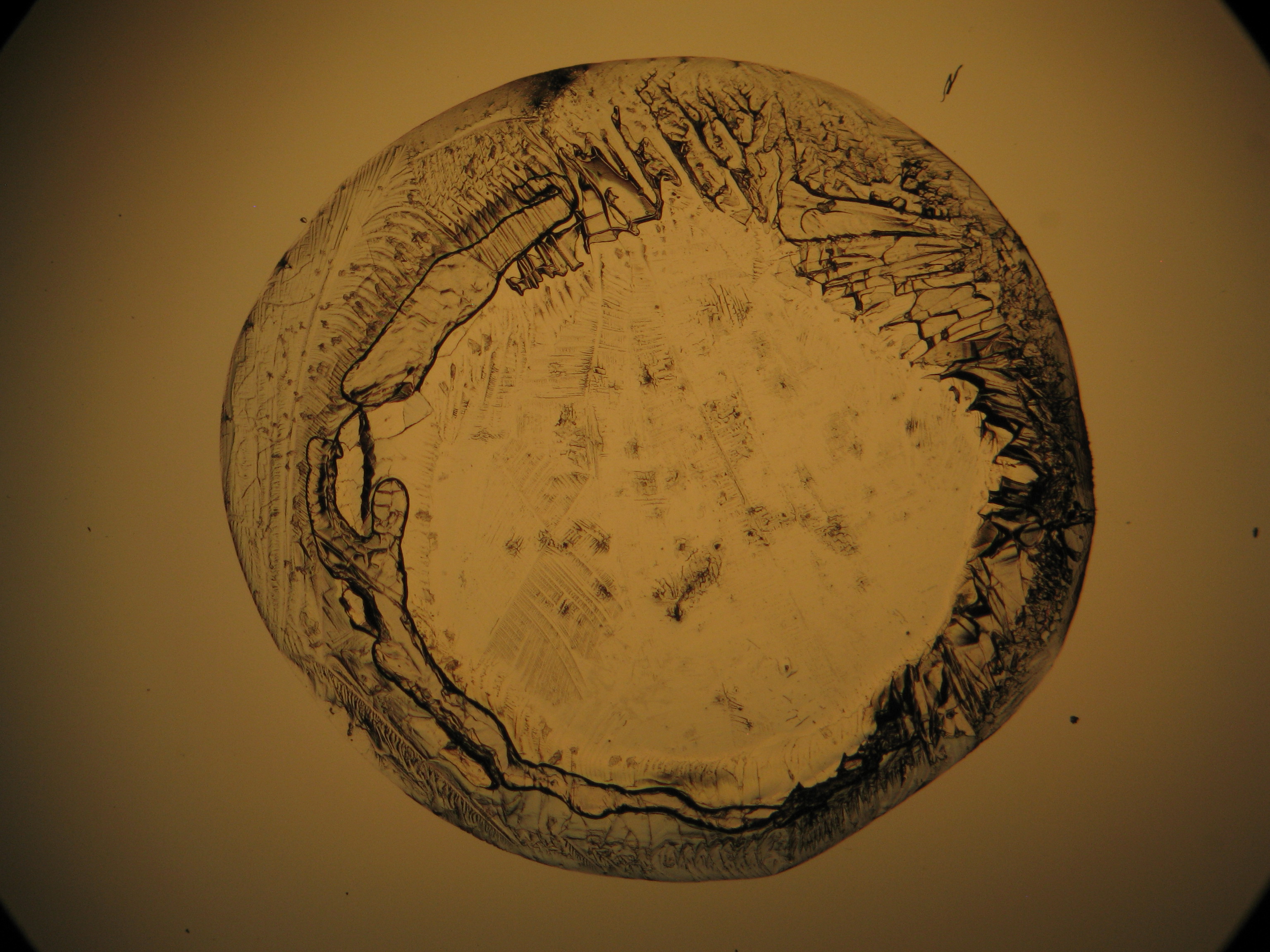
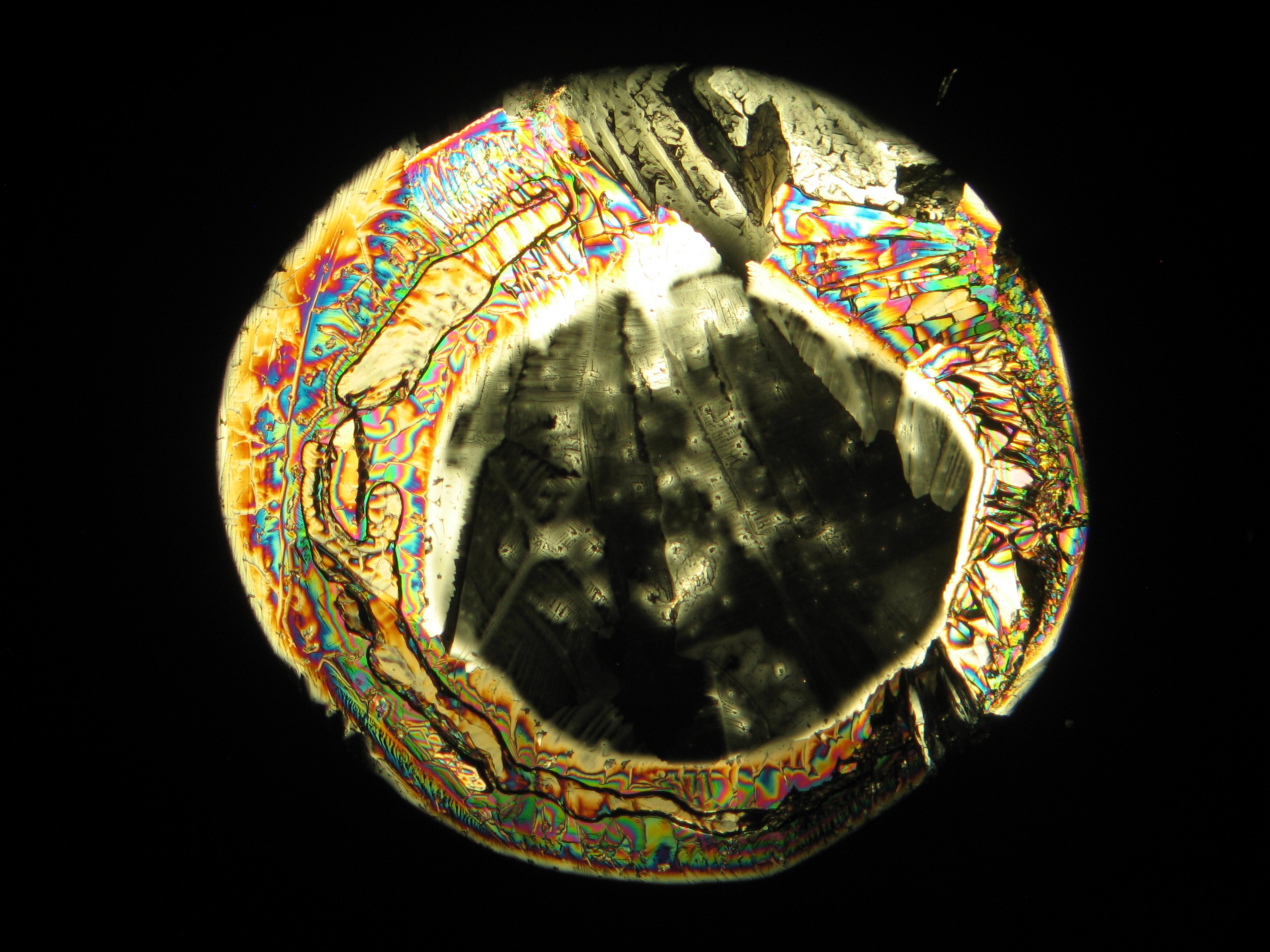
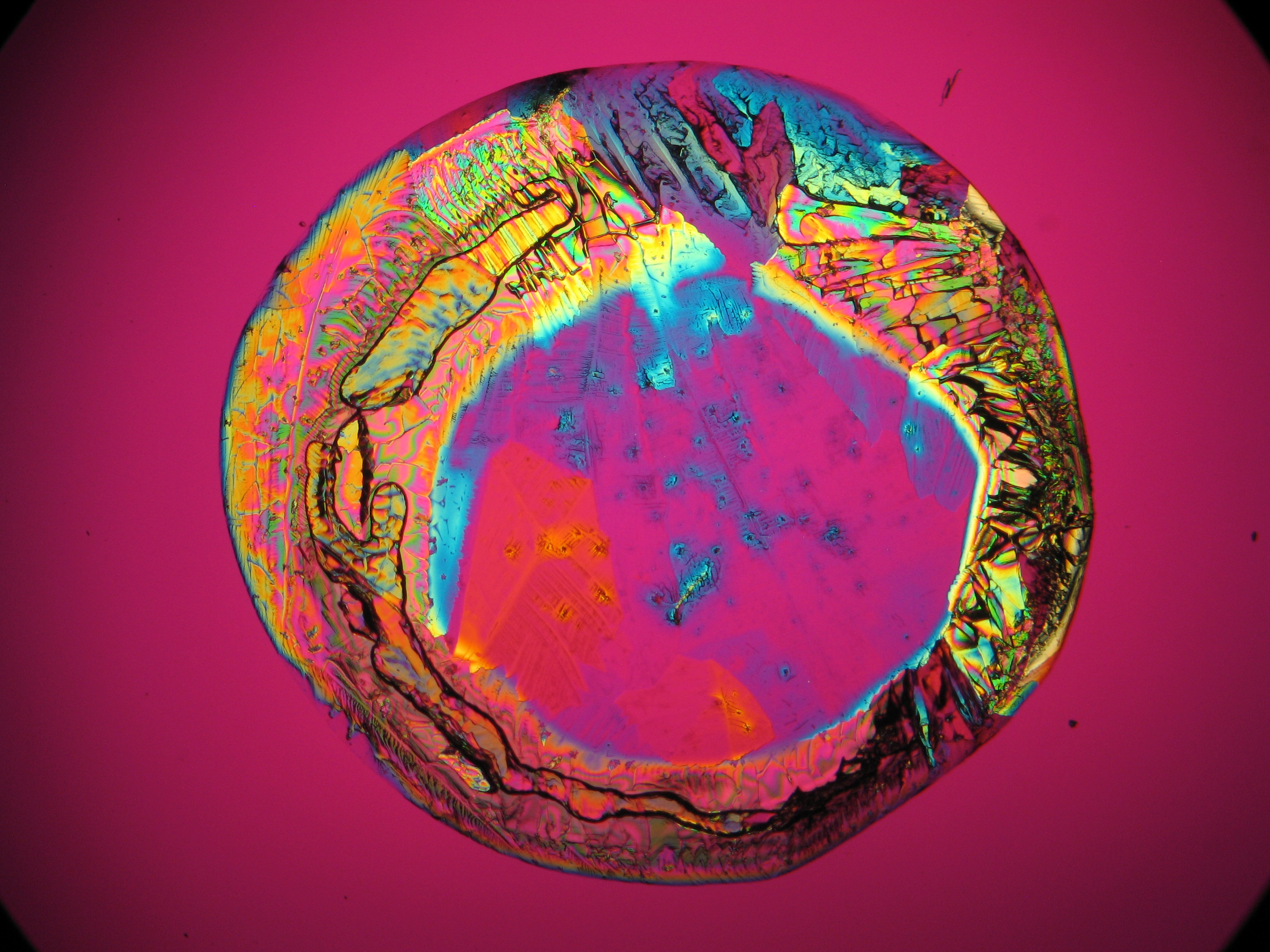
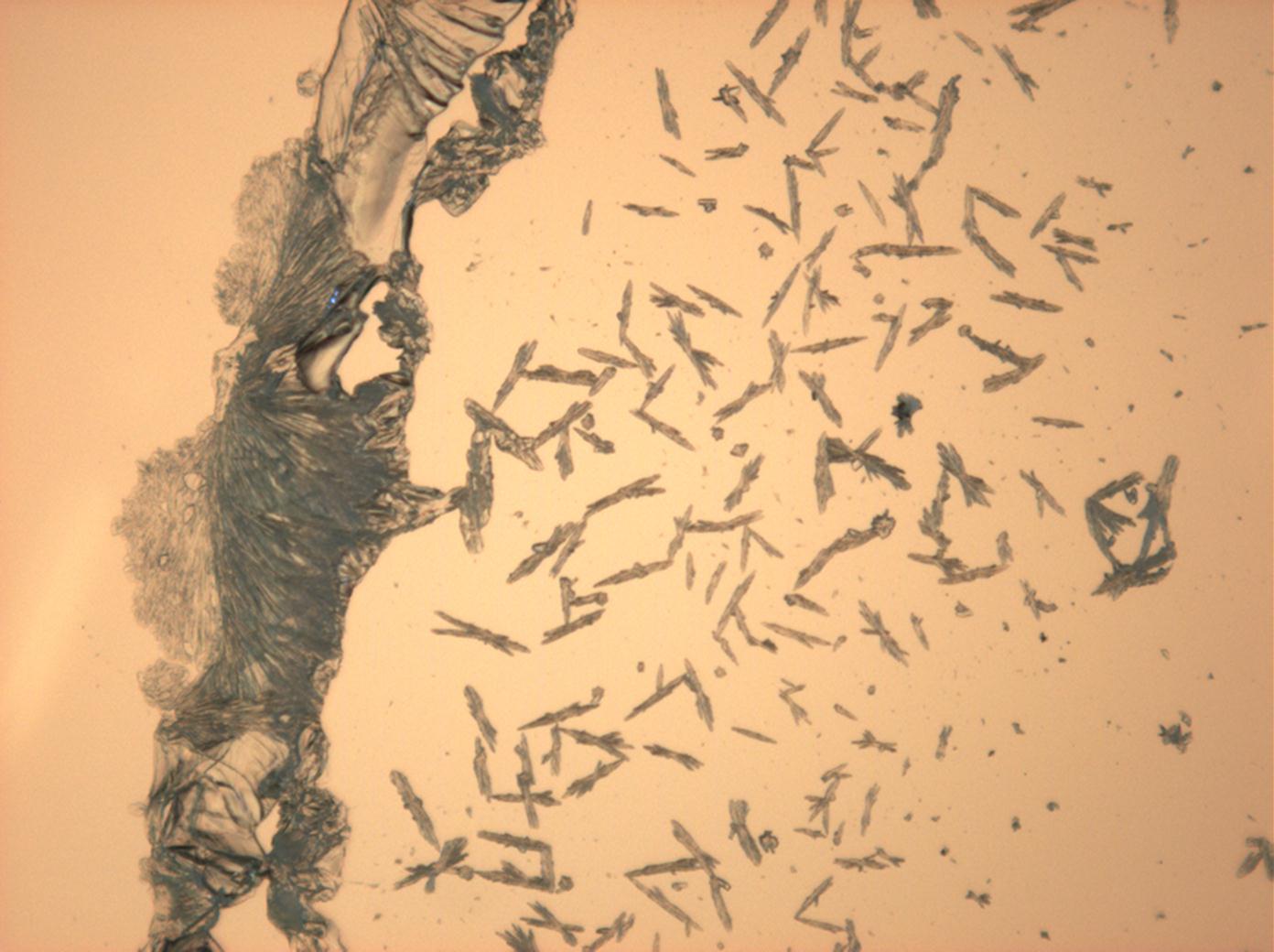
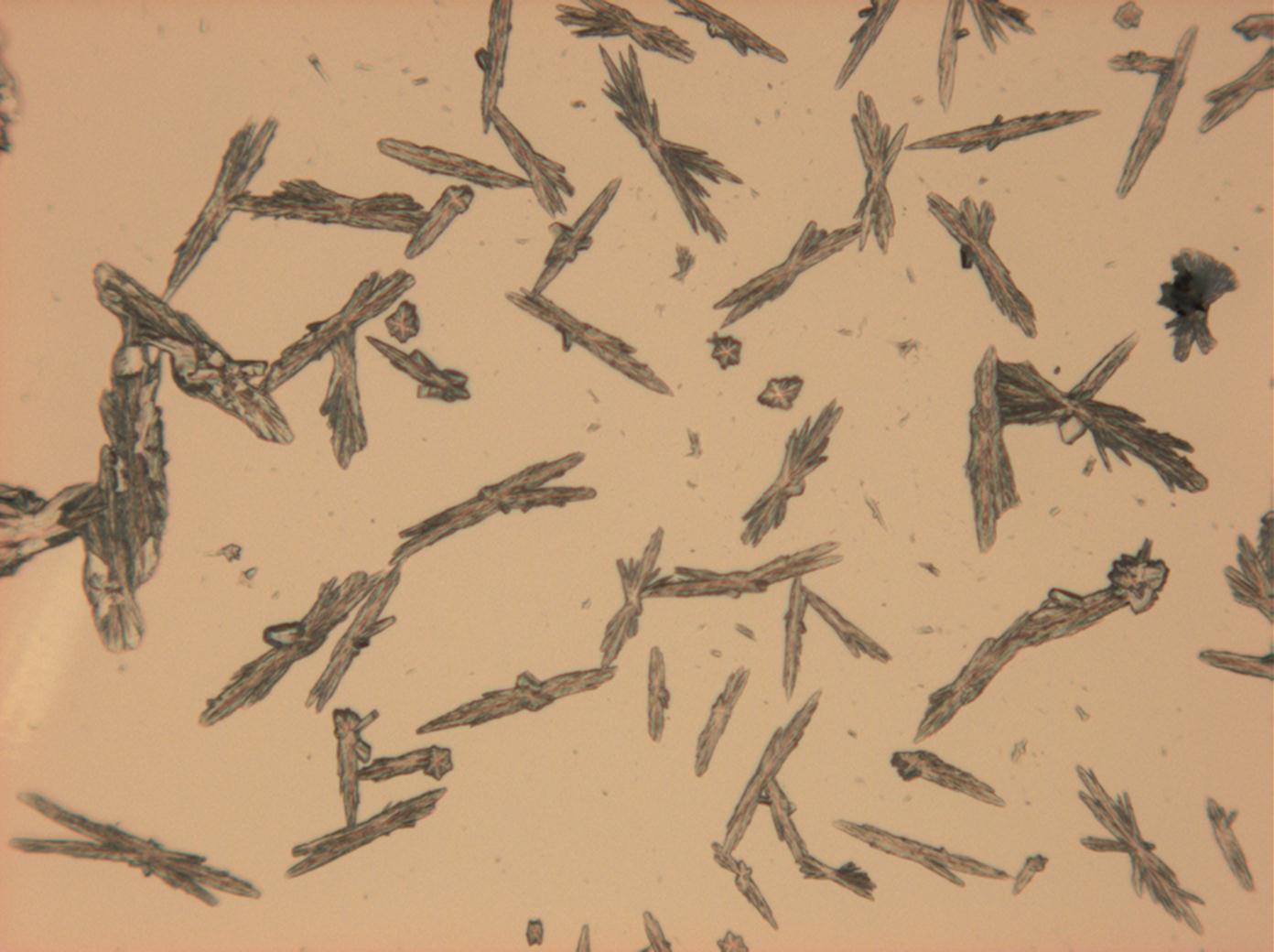
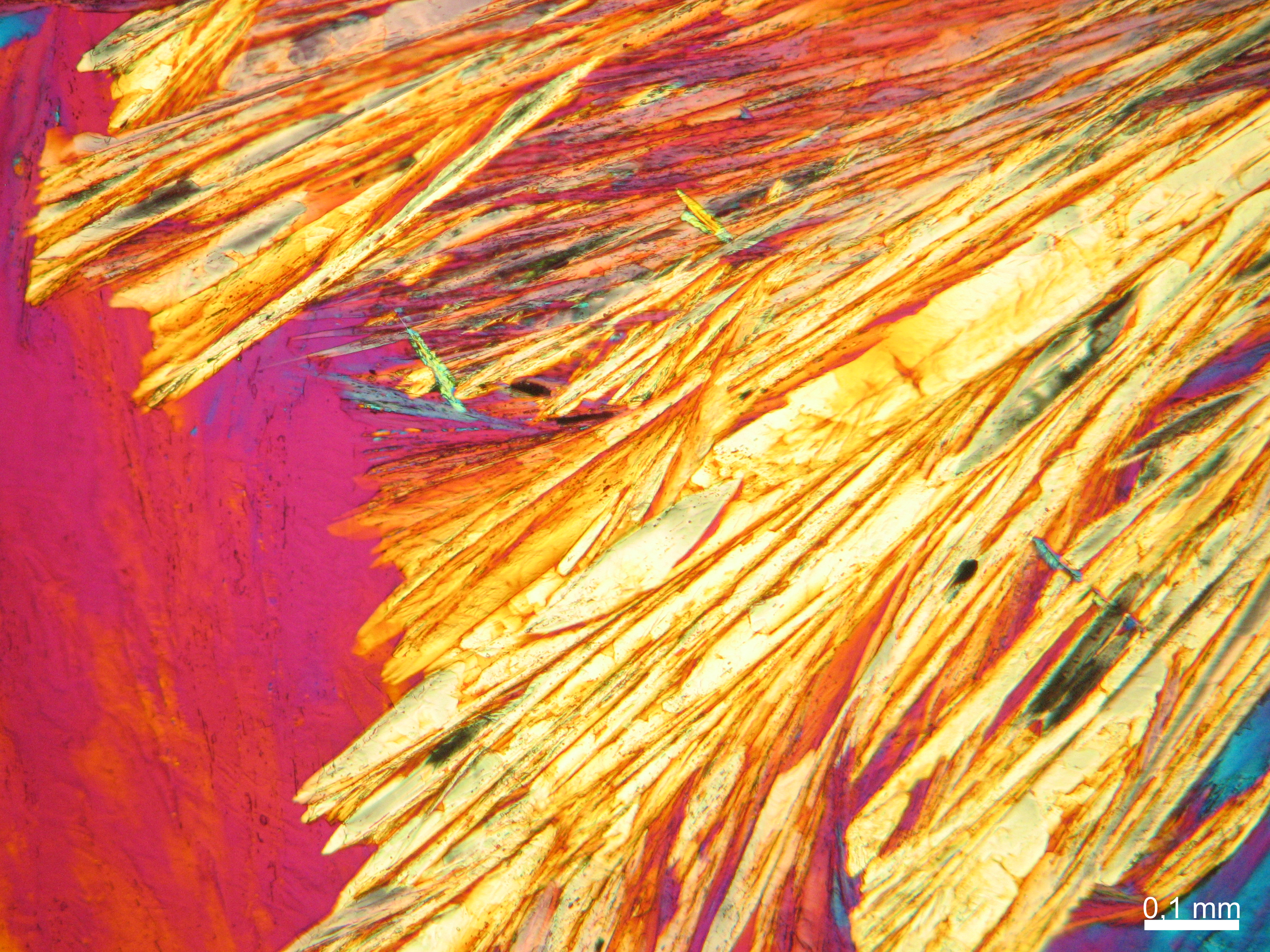
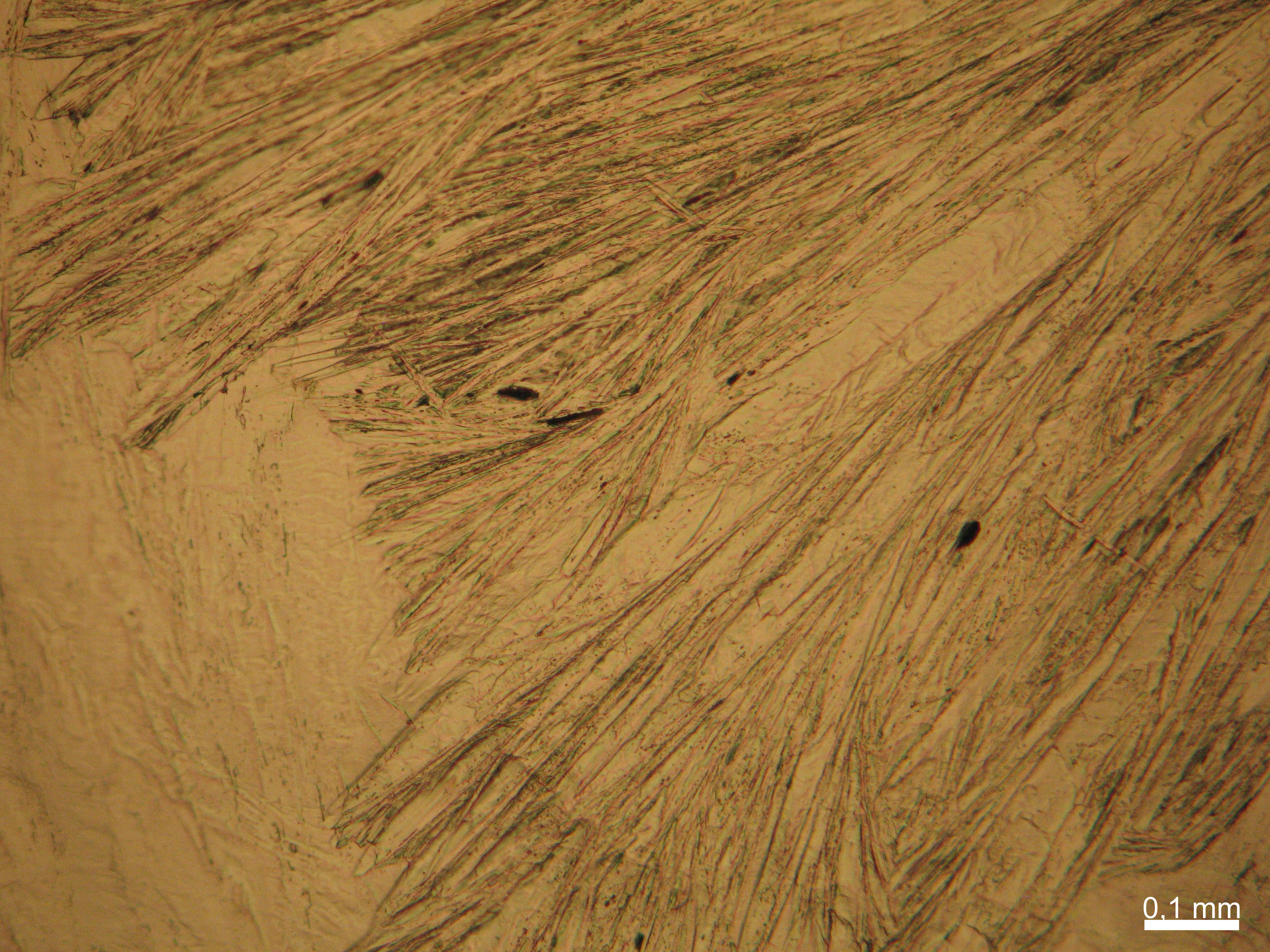
Halite (NaCl)[edit]
Figures 1-3 show halite crystals. Halite and sylvite (KCl) are the only isotropic salt crystals, i.e., belonging to the cubic crystal system. Conversely, this does not mean that all crystals that appear isotropic on the microscope slide are cubic, some salts crystallize at an orientation that makes them optically isotropic in appearance only. Therefore caution is vital. In comparison with typical cubic crystal shapes, however, the identification is unambiguous.
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Halite | NaCl | nD=1.5443 | cubic | isotropic |
- Halite, crystallized from aqueous solution on a microscope slide
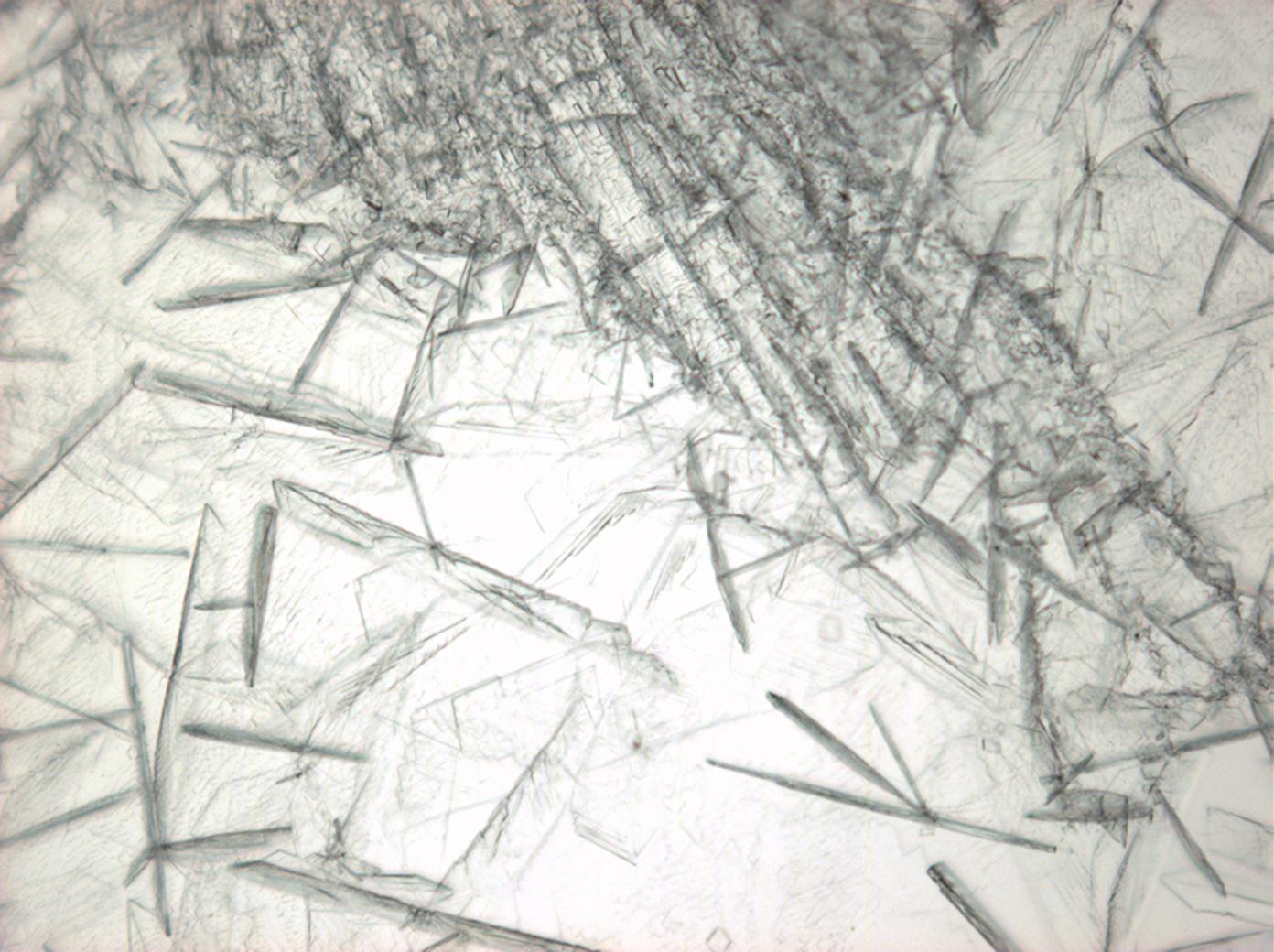
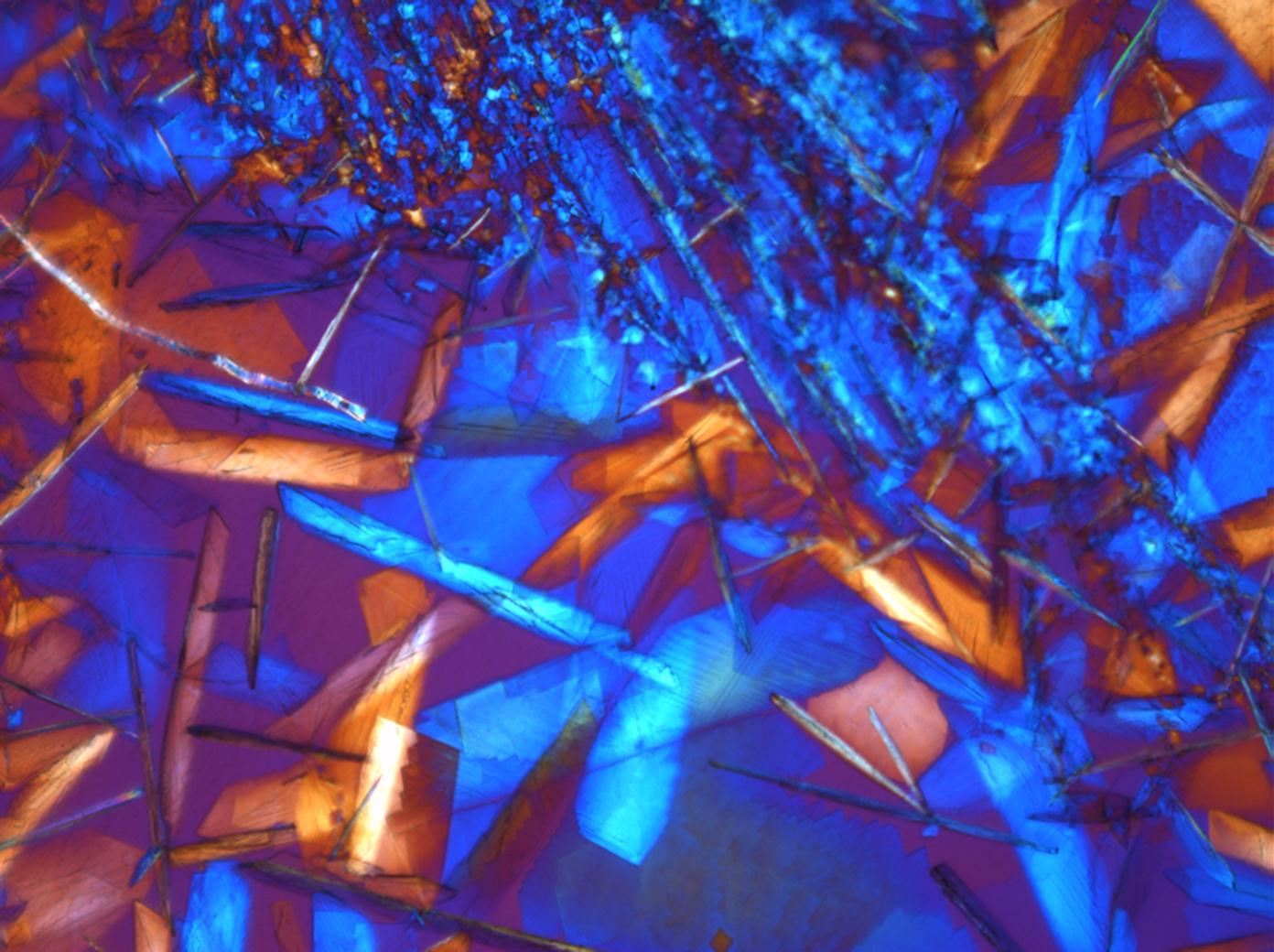
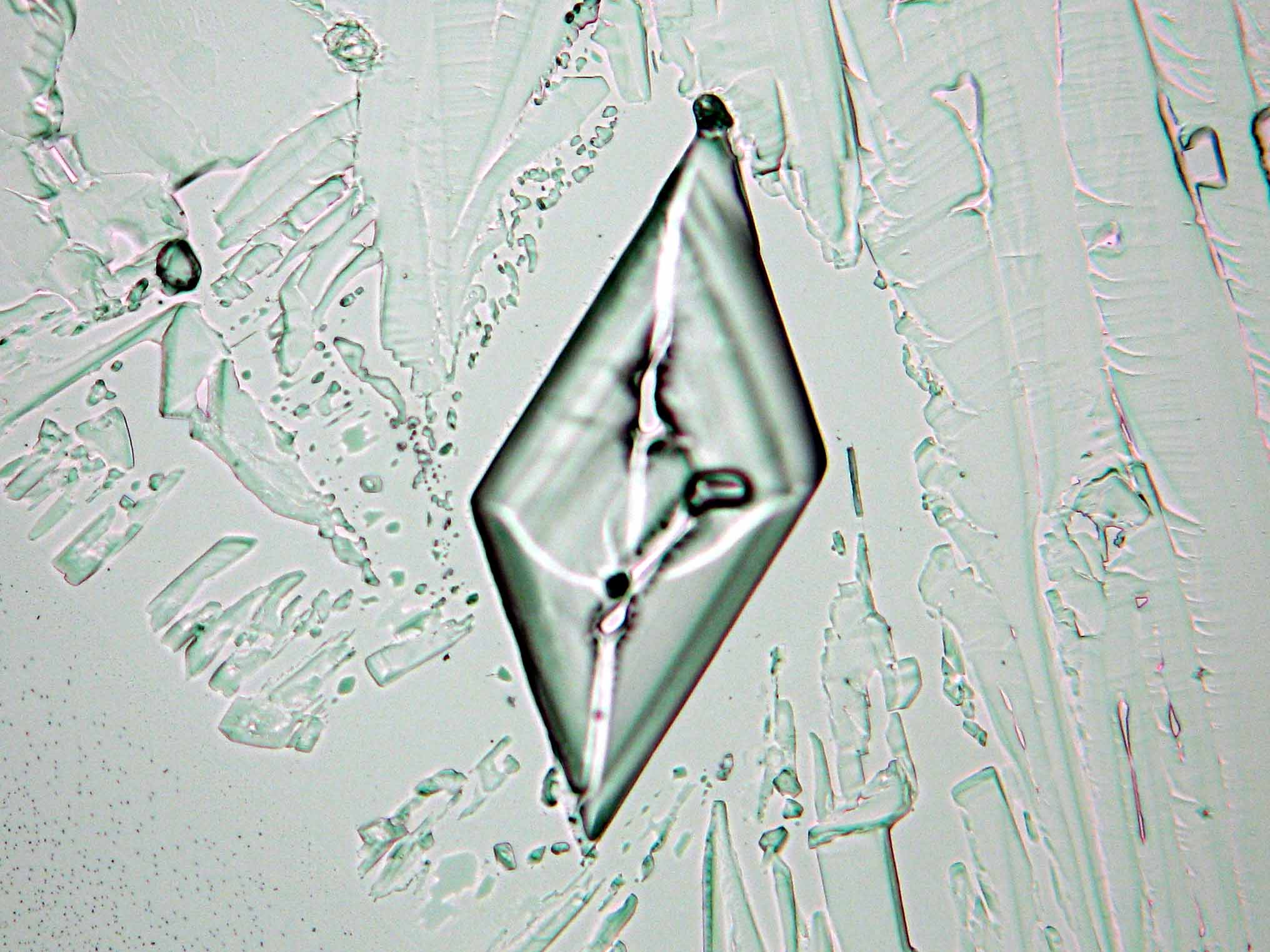
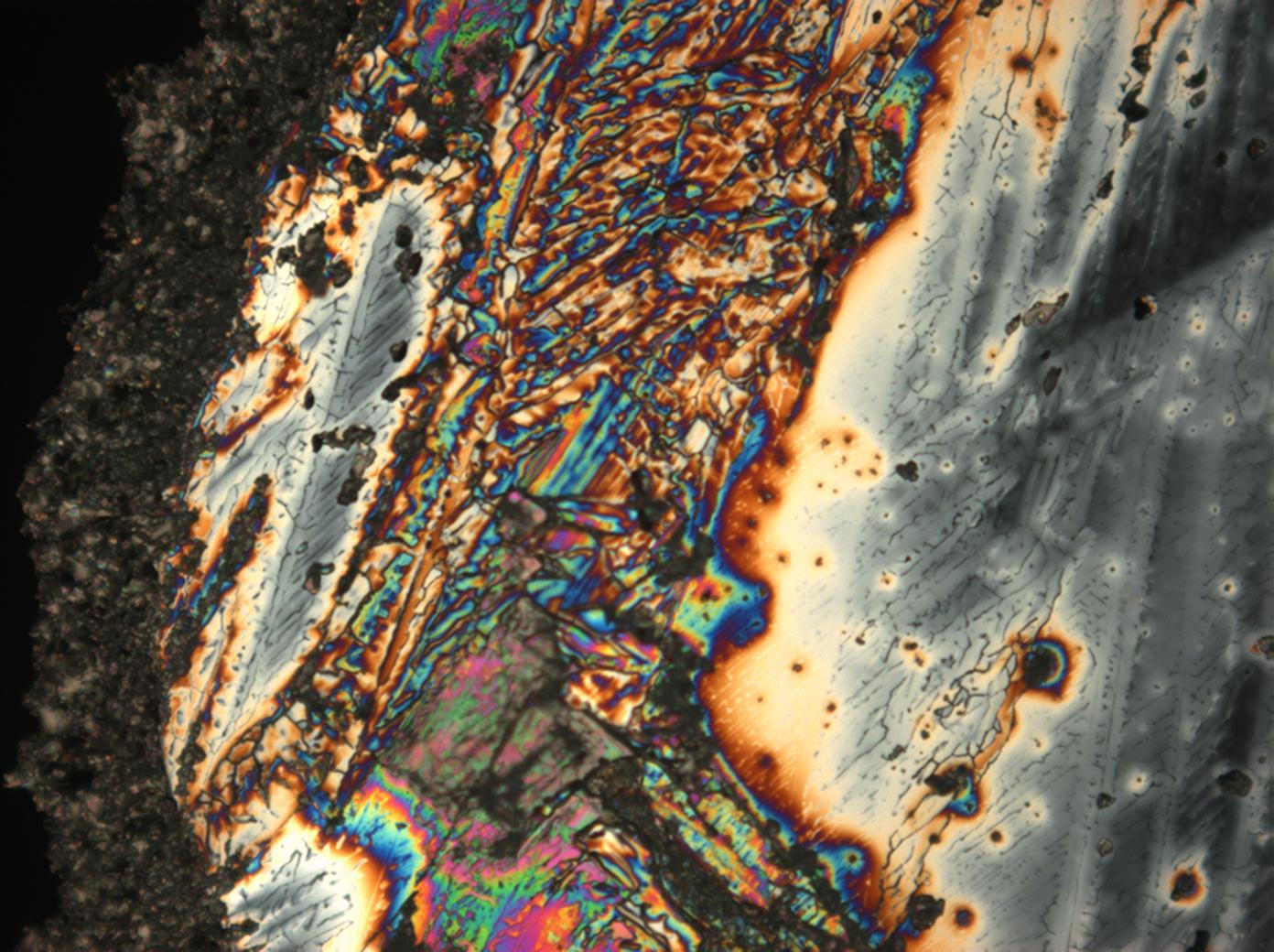
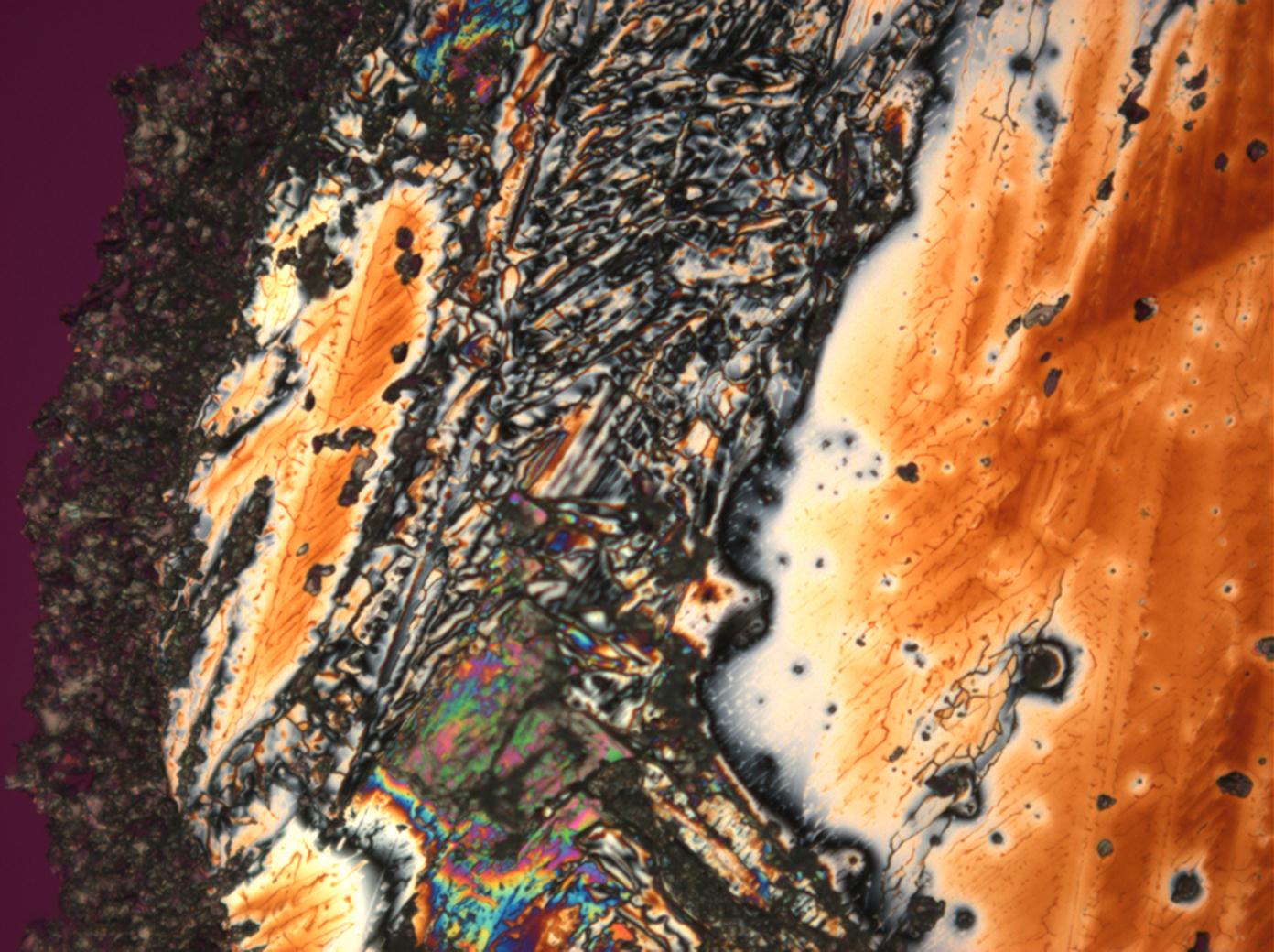
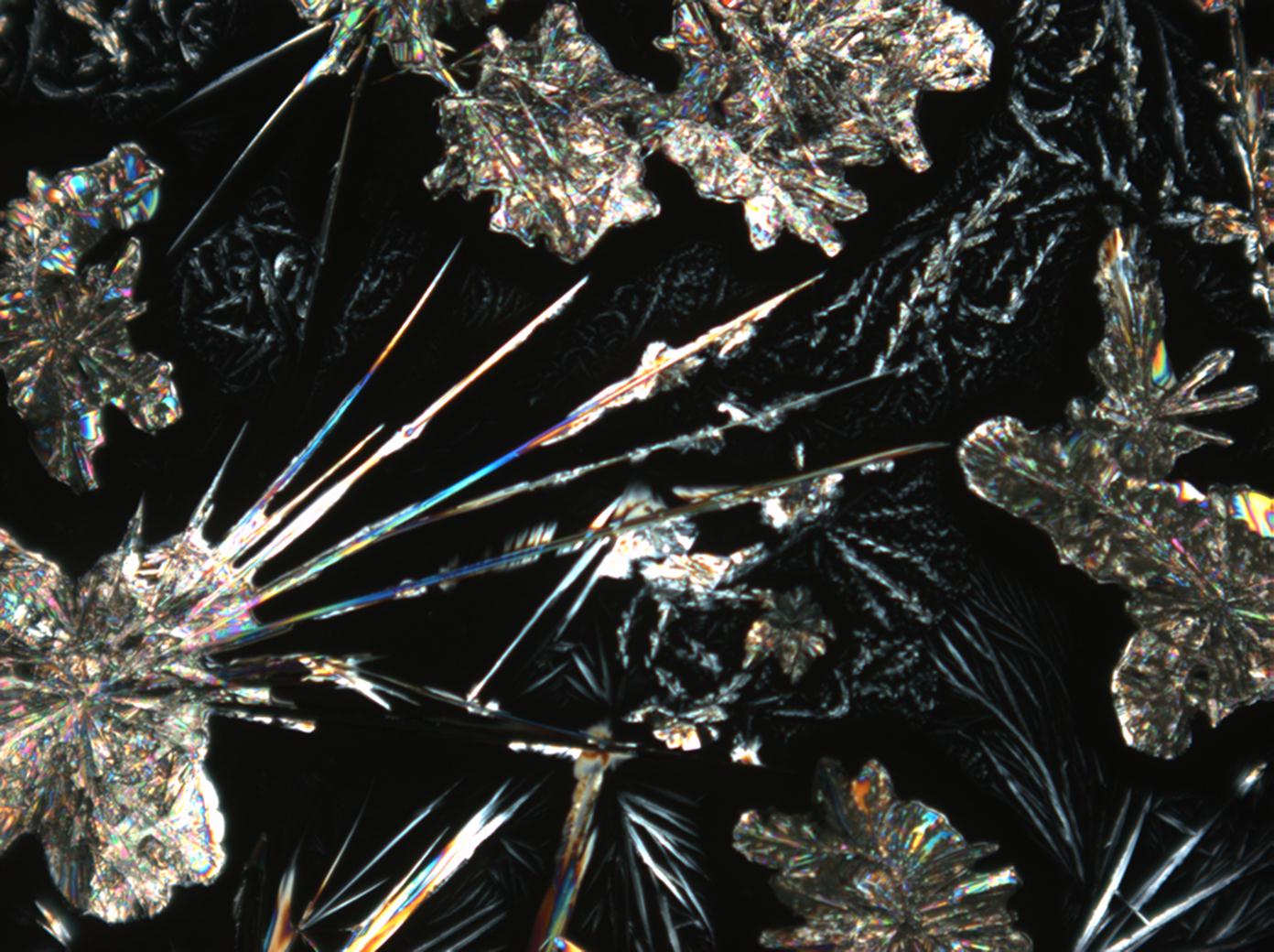
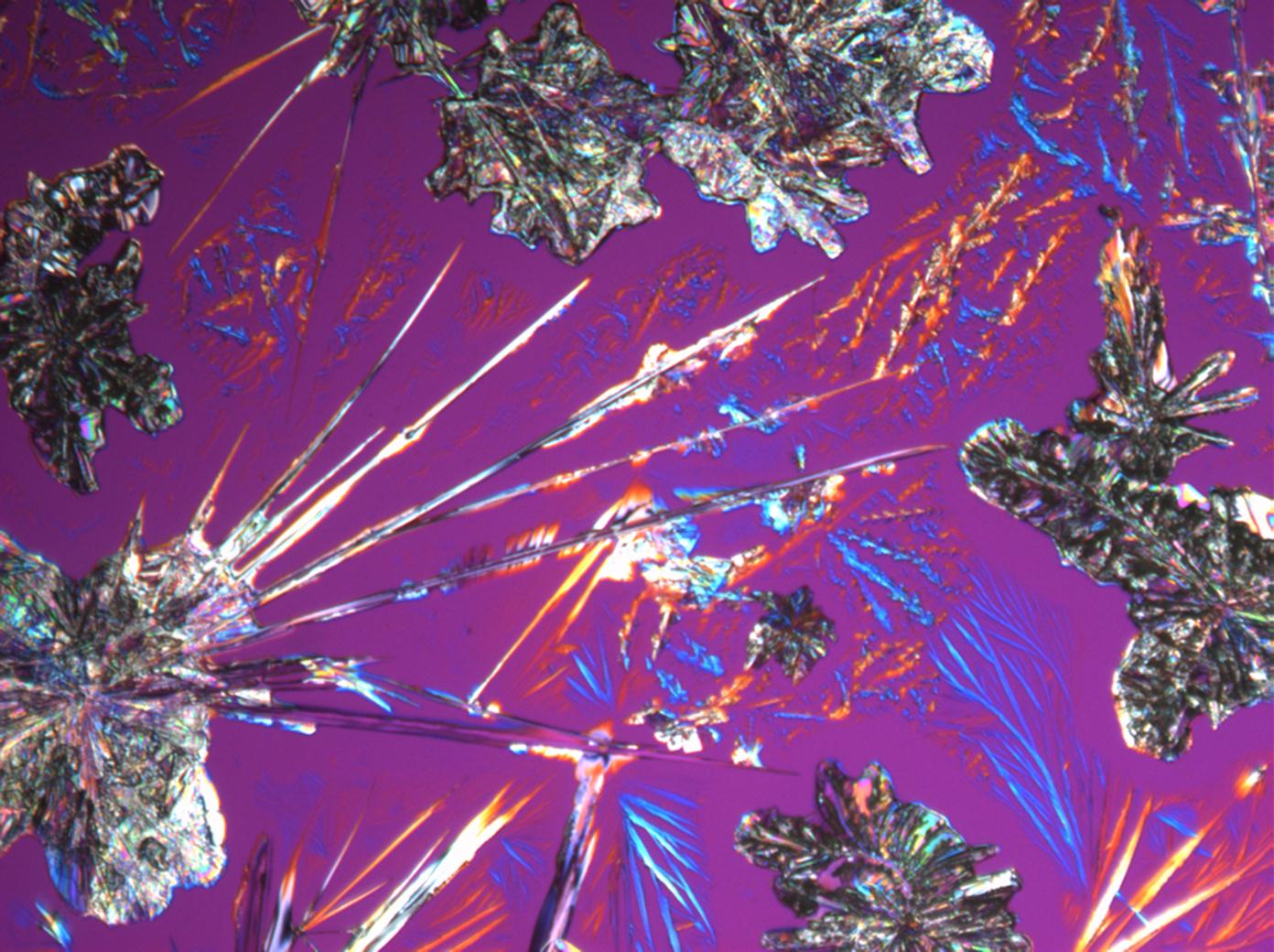
Calcium chloride[edit]
Calcium chloride only crystallizes at relatively low relative humidity levels (pure salts at a RH <30, 8% and 20°C). Because the relative humidity on objects and in the laboratory usually lies above this value, the crystalline form of calcium chloride can only rarely be seen on an object. It is possible to observe the crystals under the polarizing microscope, by warming up the slide until they form. However, the crystals dissolve in the ambient air when the temperature cools down and the RH rises. Figures 4- 6 show calcium chloride crystals while warming.
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Antarcticite | CaCl2•6H2O | Δ= 0.024 | no =1.417-1.494 ne = 1.393-1.550 |
trigonal | negative |
- Calcium chloride, crystallized from aqueous solution on a microscope slide
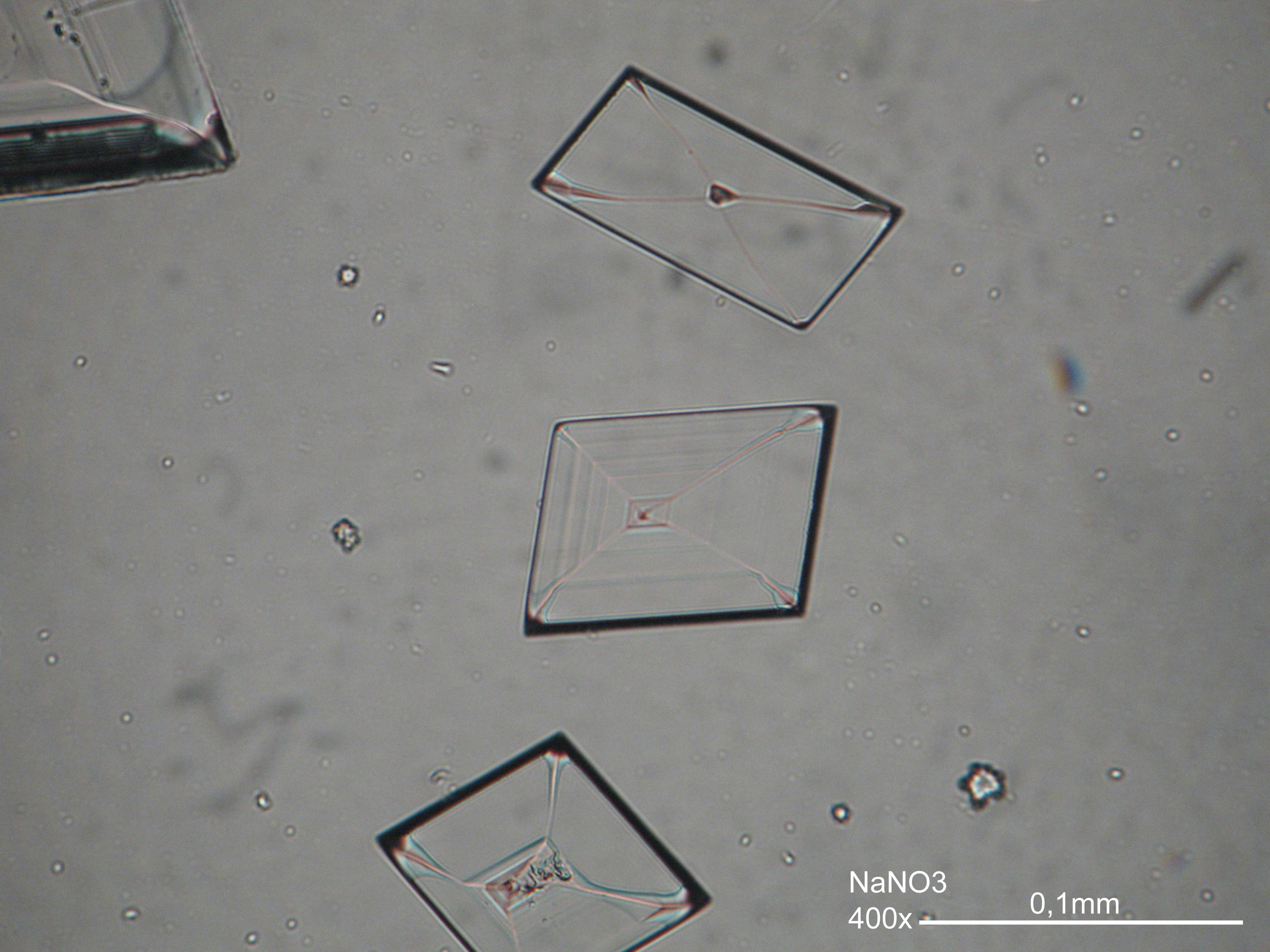
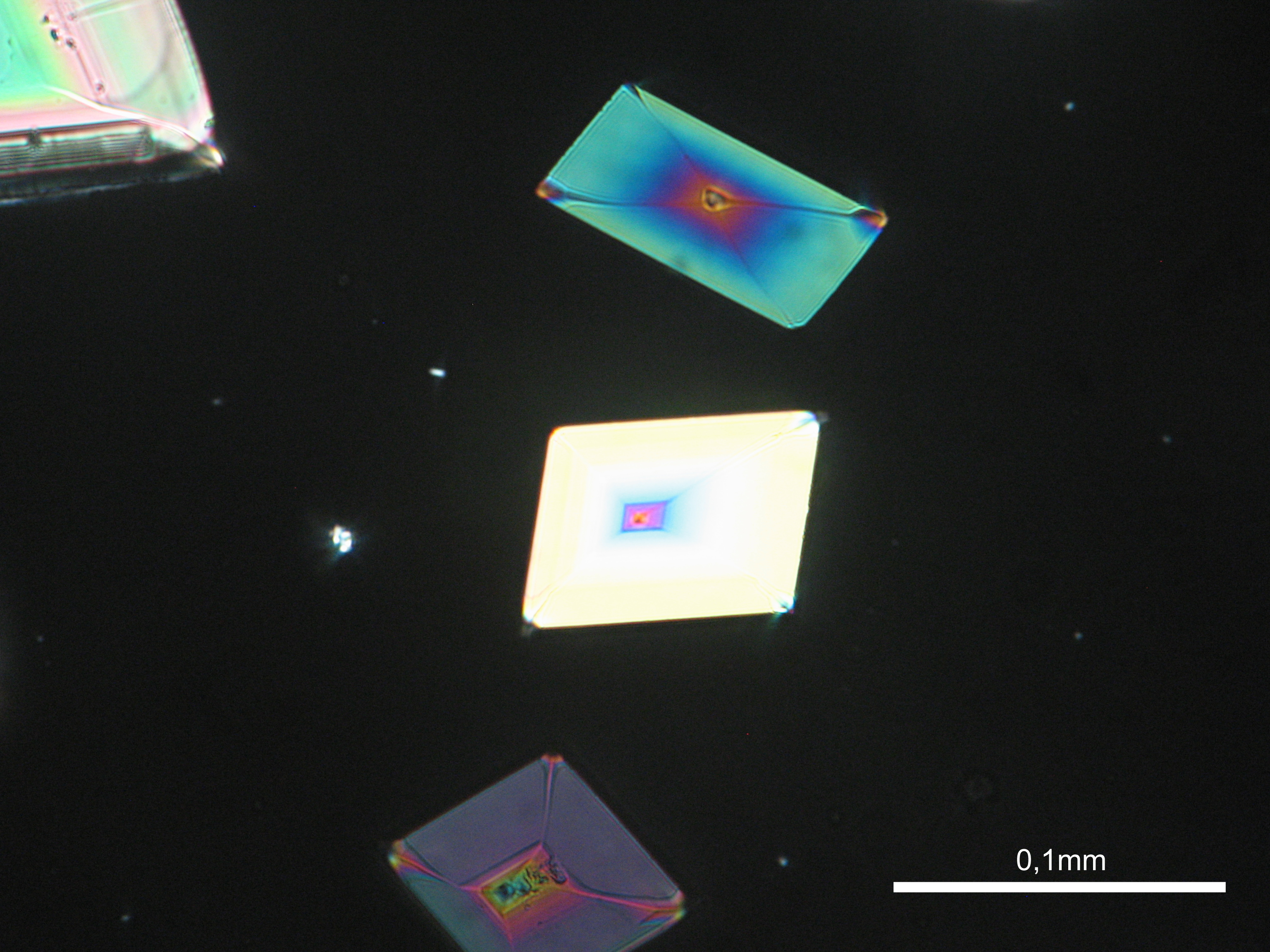
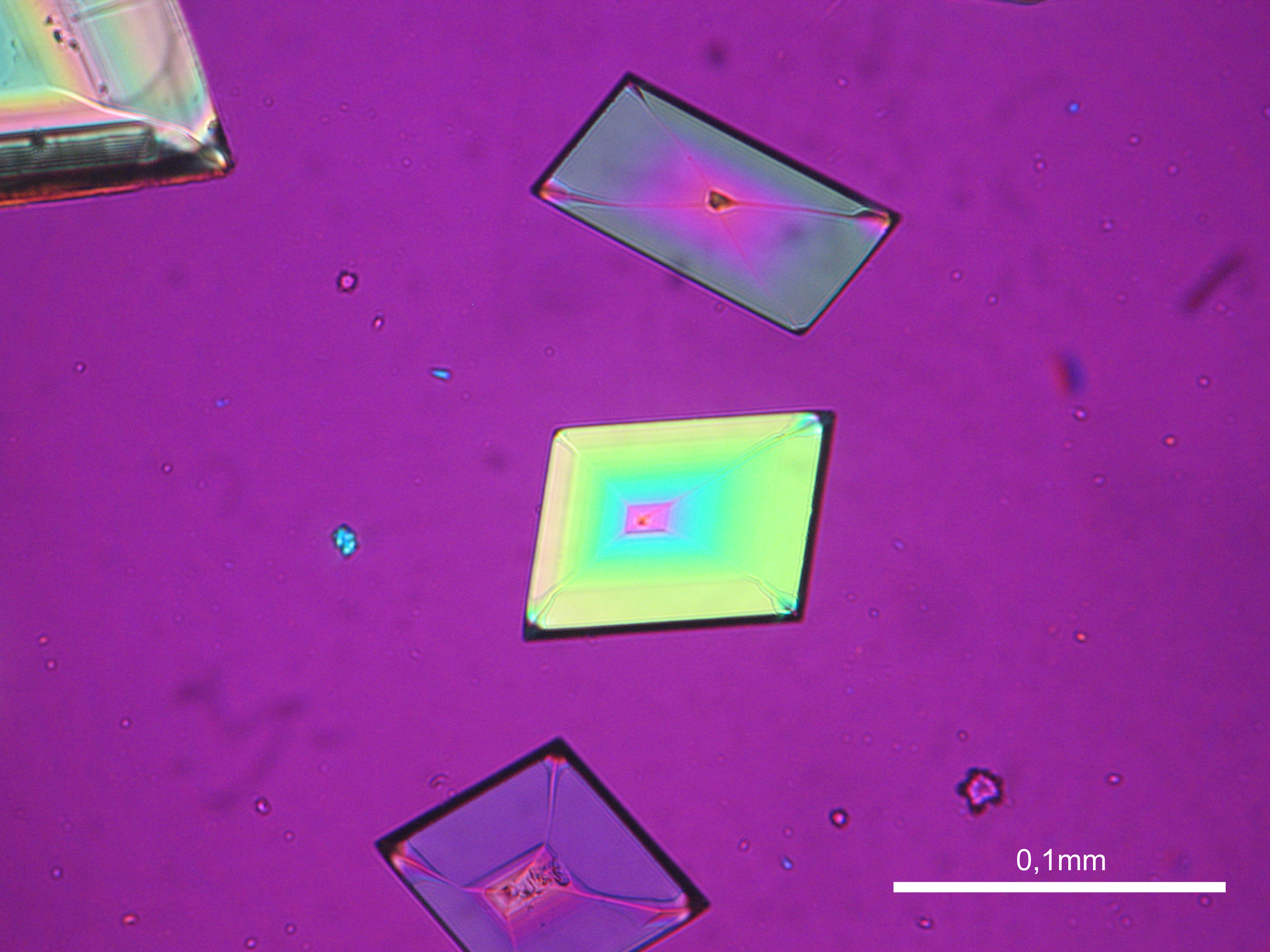
Niter[edit]
| Salt | Chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Niter | KNO3 | Δ = 0.171 | α = 1.335 β = 1.505 γ = 1.506 |
orthorhombic | biaxial negative |
- Niter crystallized from aqueous solution on a microscope slide
Calcium nitrate[edit]
| Salt | Chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Nitrocalcite |
- Calcium nitrate crystallized from aqueous solution on a microscope slide
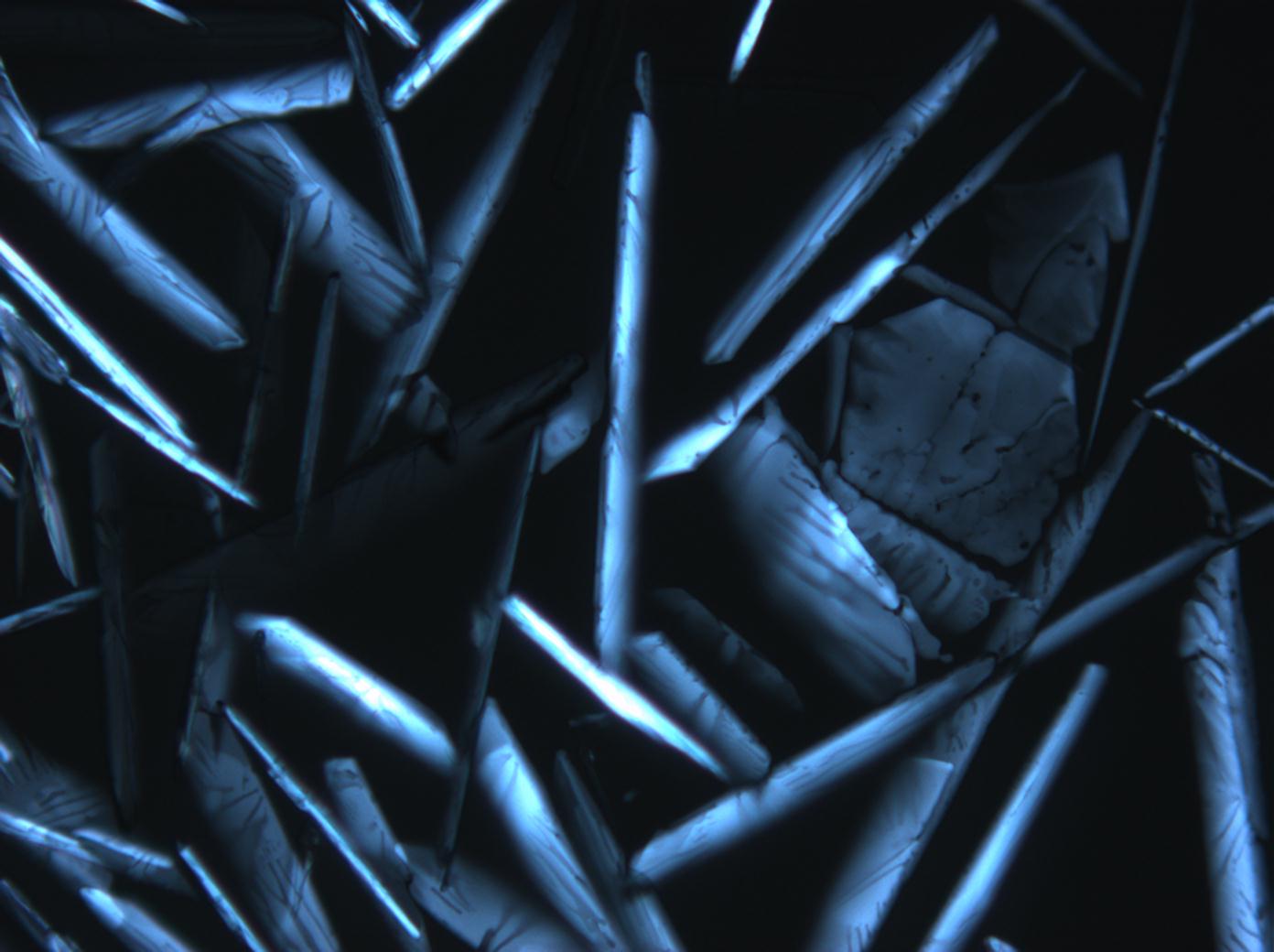
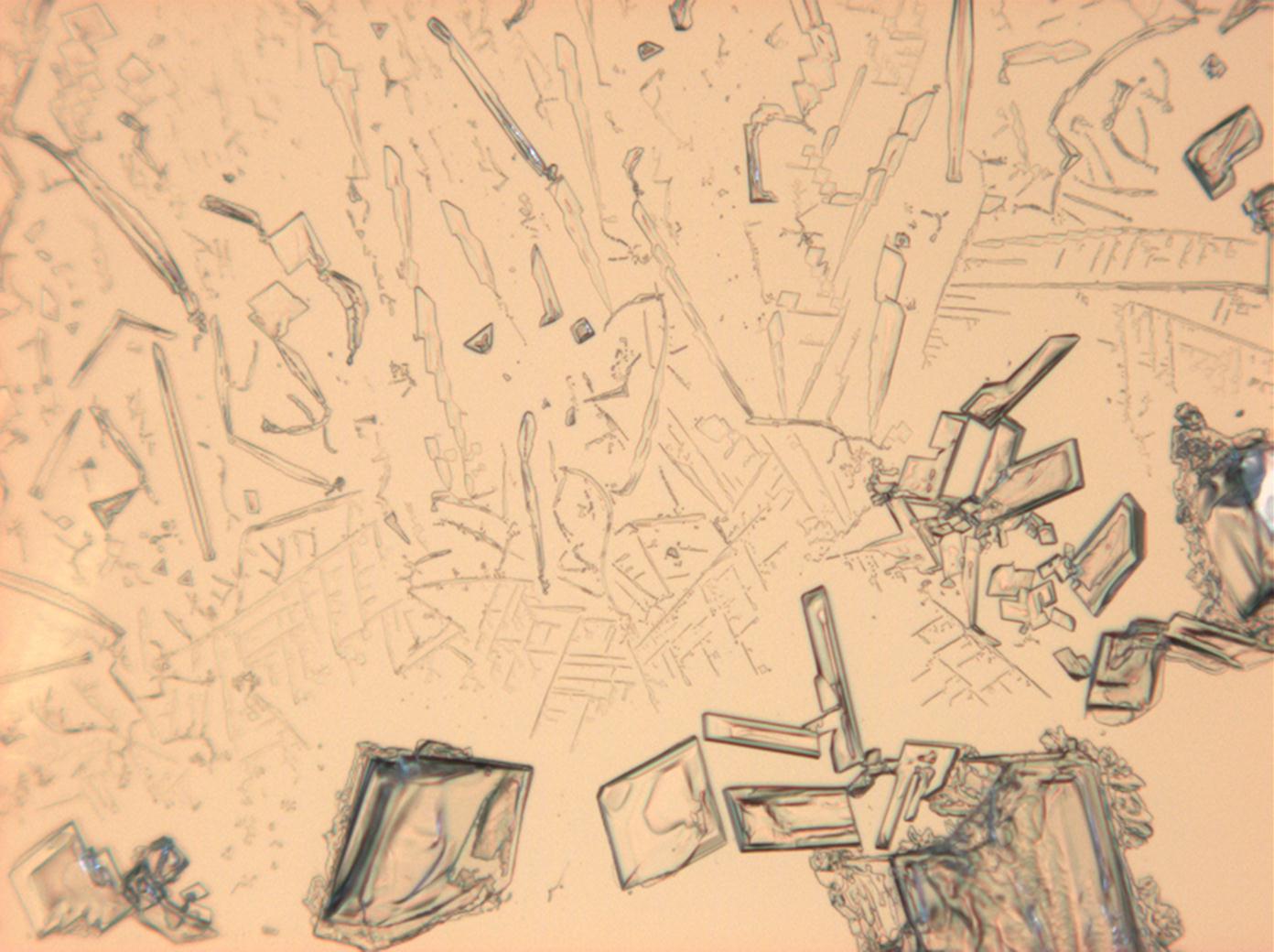
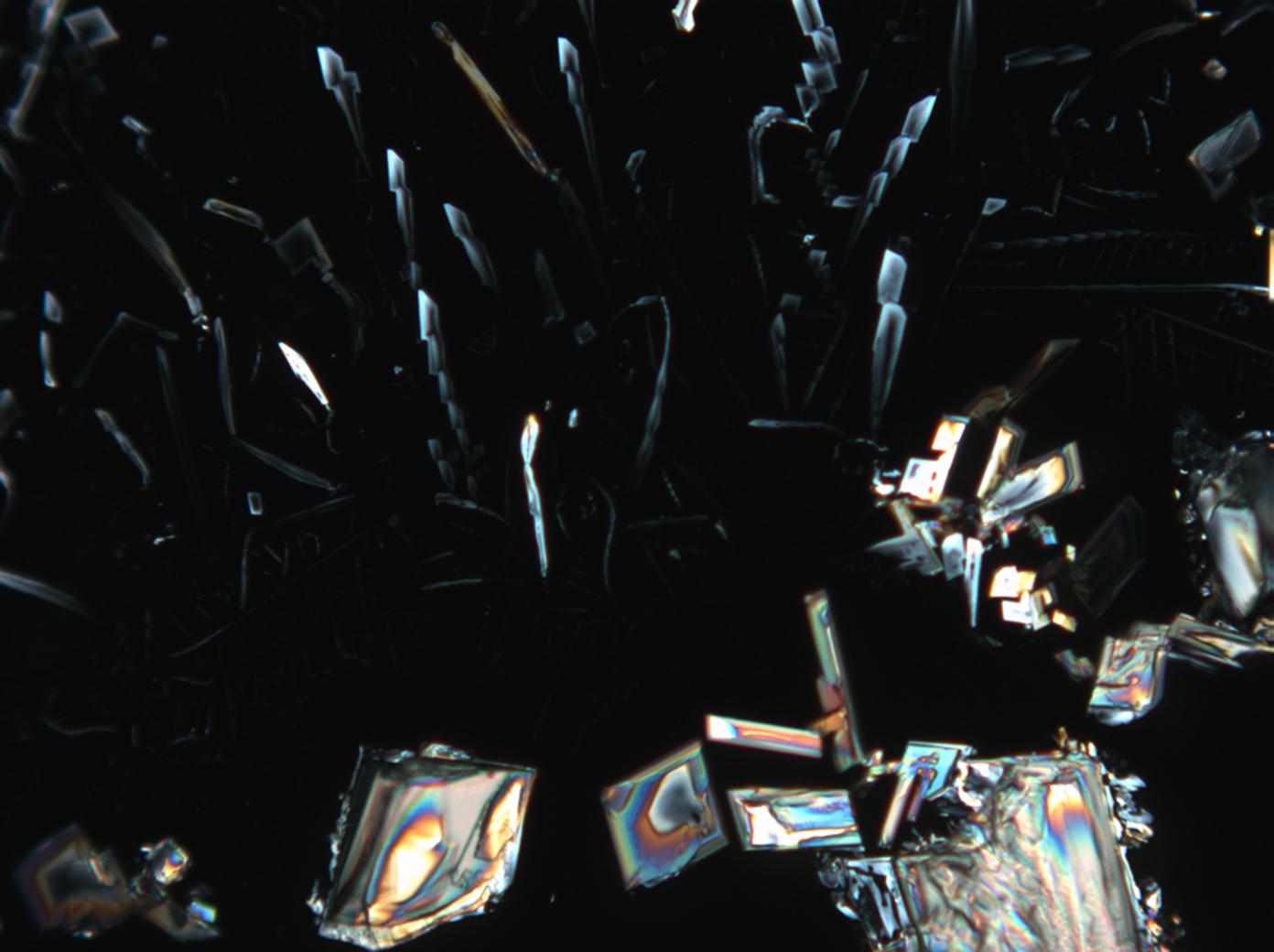
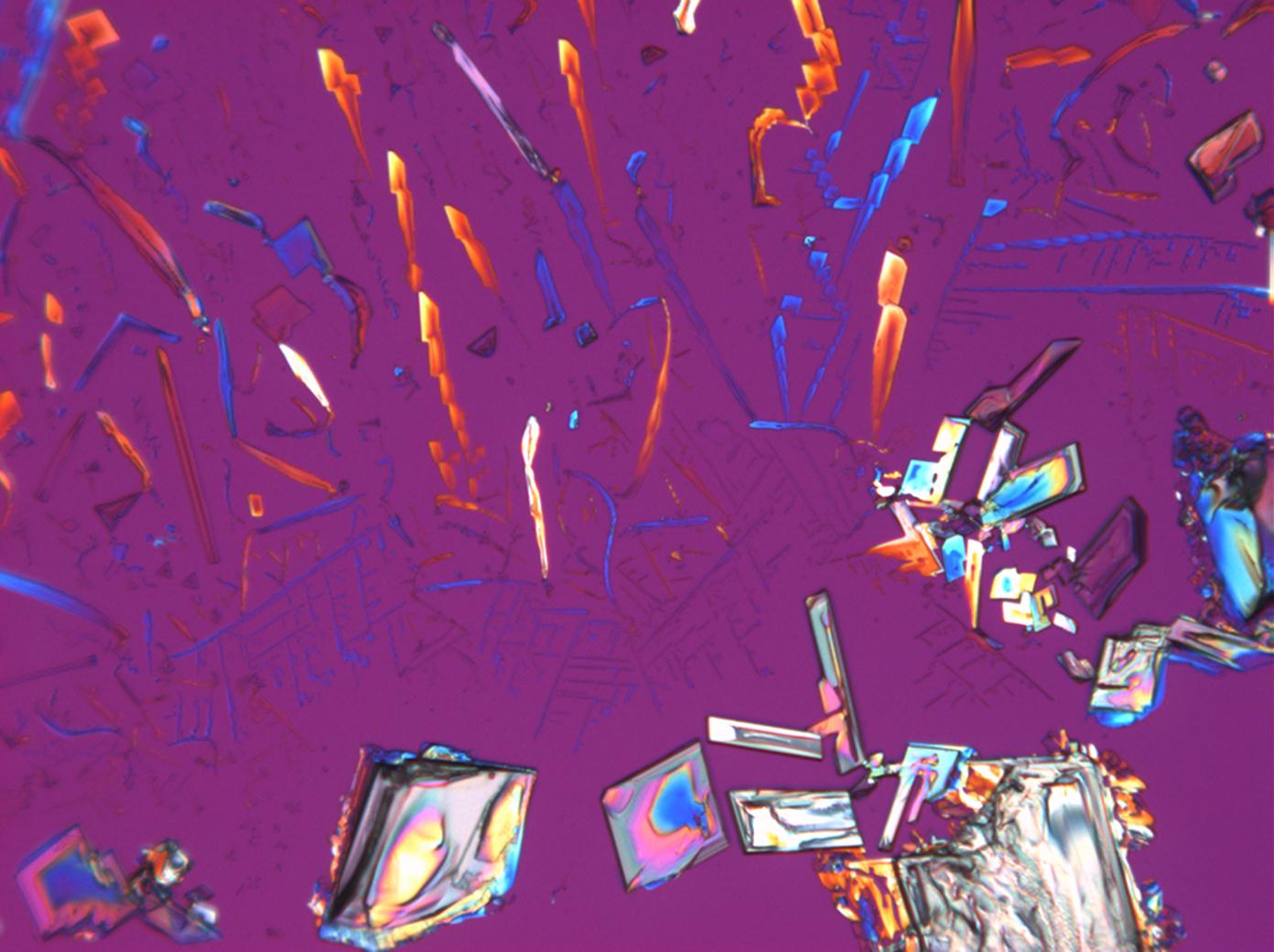
Magnesium nitrate[edit]
| Salt | Chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Nitromagnesite | Mg(NO3)2•6H2O | Δ = 0.166 | nx = 1.34 ny = 1.506 nz = 1.506 |
monoclinic | negative |
- Magnesium nitrate crystallized from aqueous solution on a microscope slide
- Mg-NO3-2 (1).jpg Figure 14:Magnesium nitrate kristalle unter polarisiertem Licht mit Analysator und Rot I
Gypsum[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Gypsum | CaSO4•2H2O | Δ = 0.0092 | α = 1.5207 β = 1.5230 γ = 1.5299 |
monoclinic | biaxial positive |
- Gypsum crystallized from aqueous solution on a microscope slide
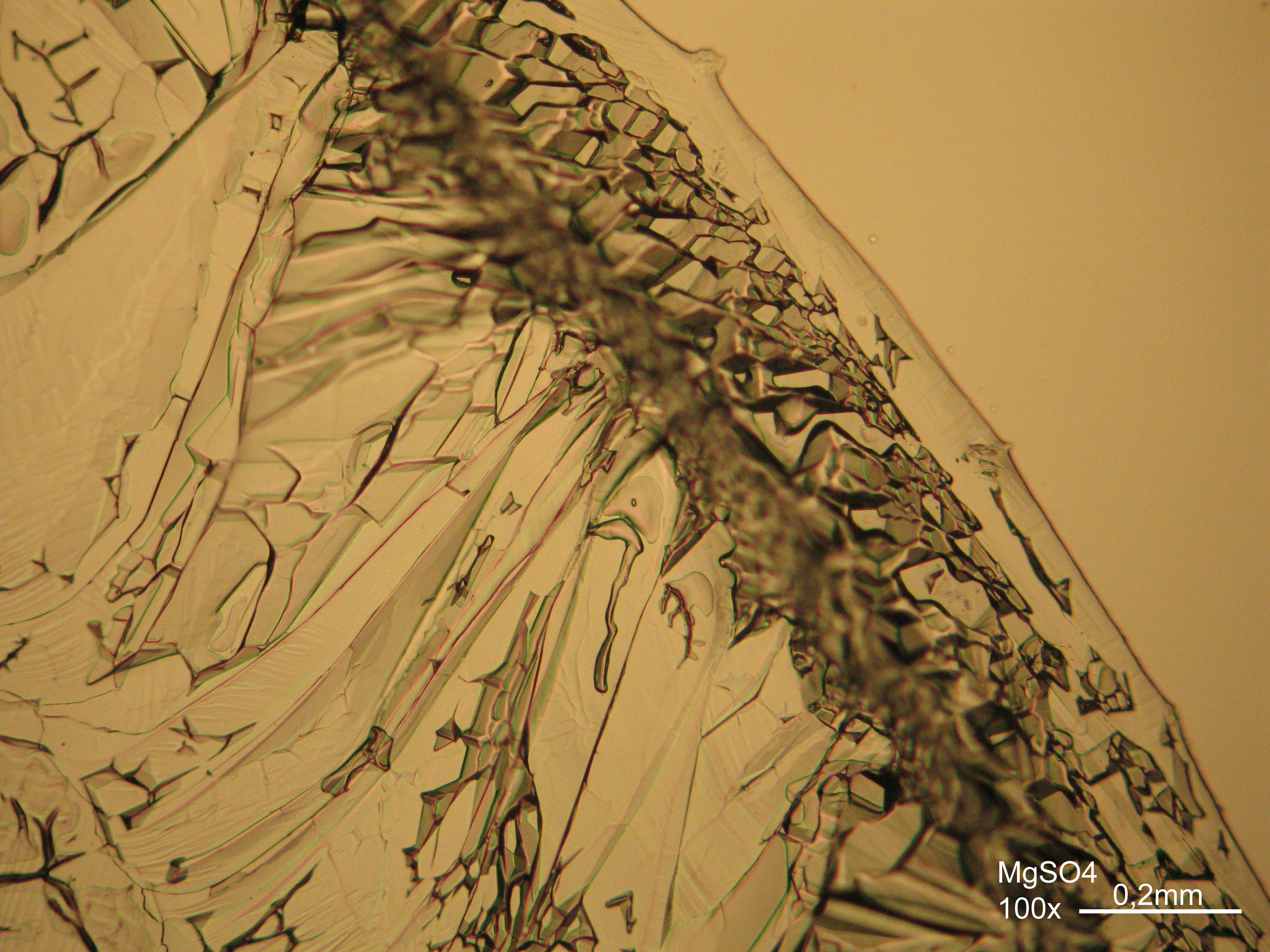
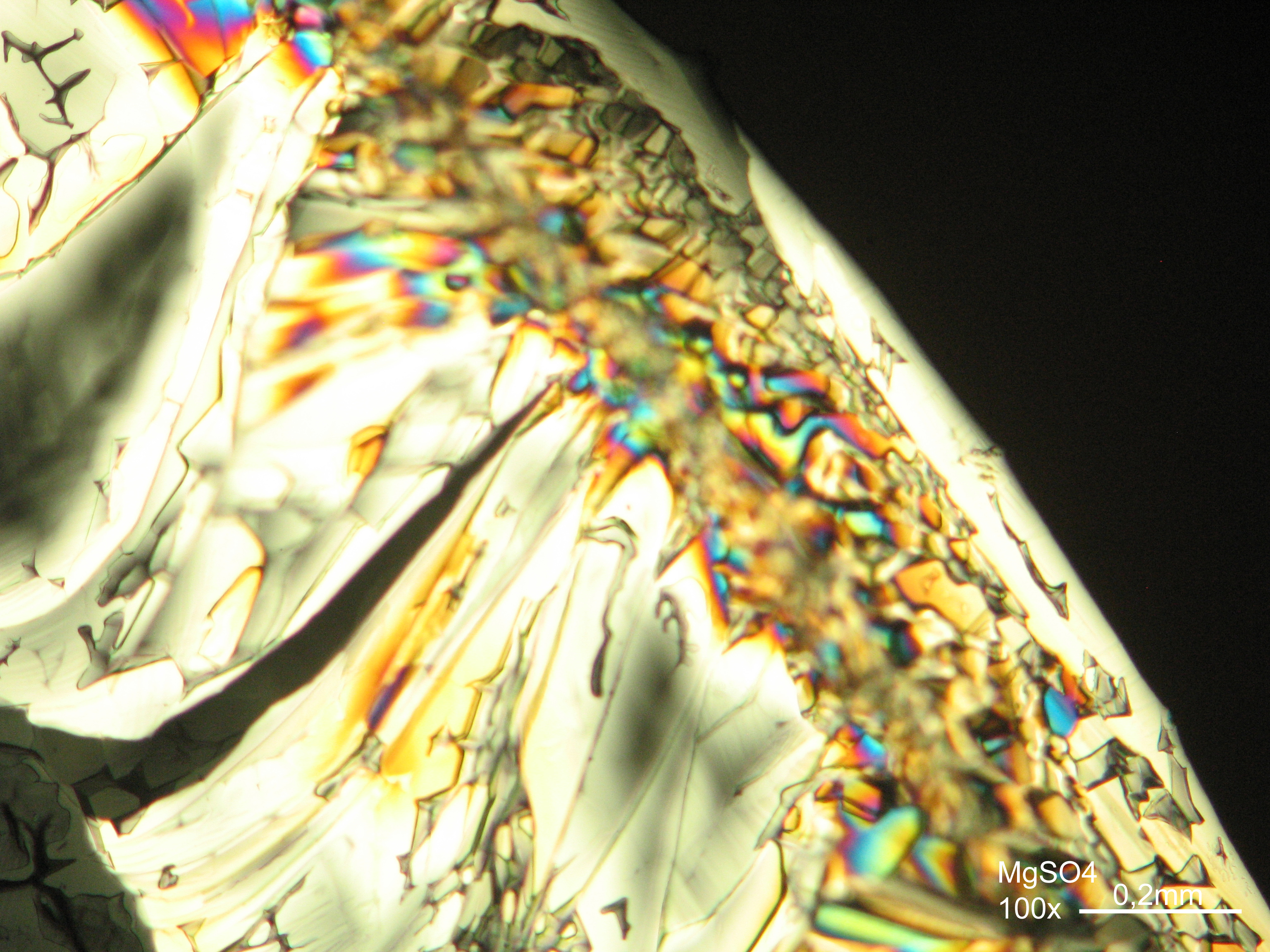
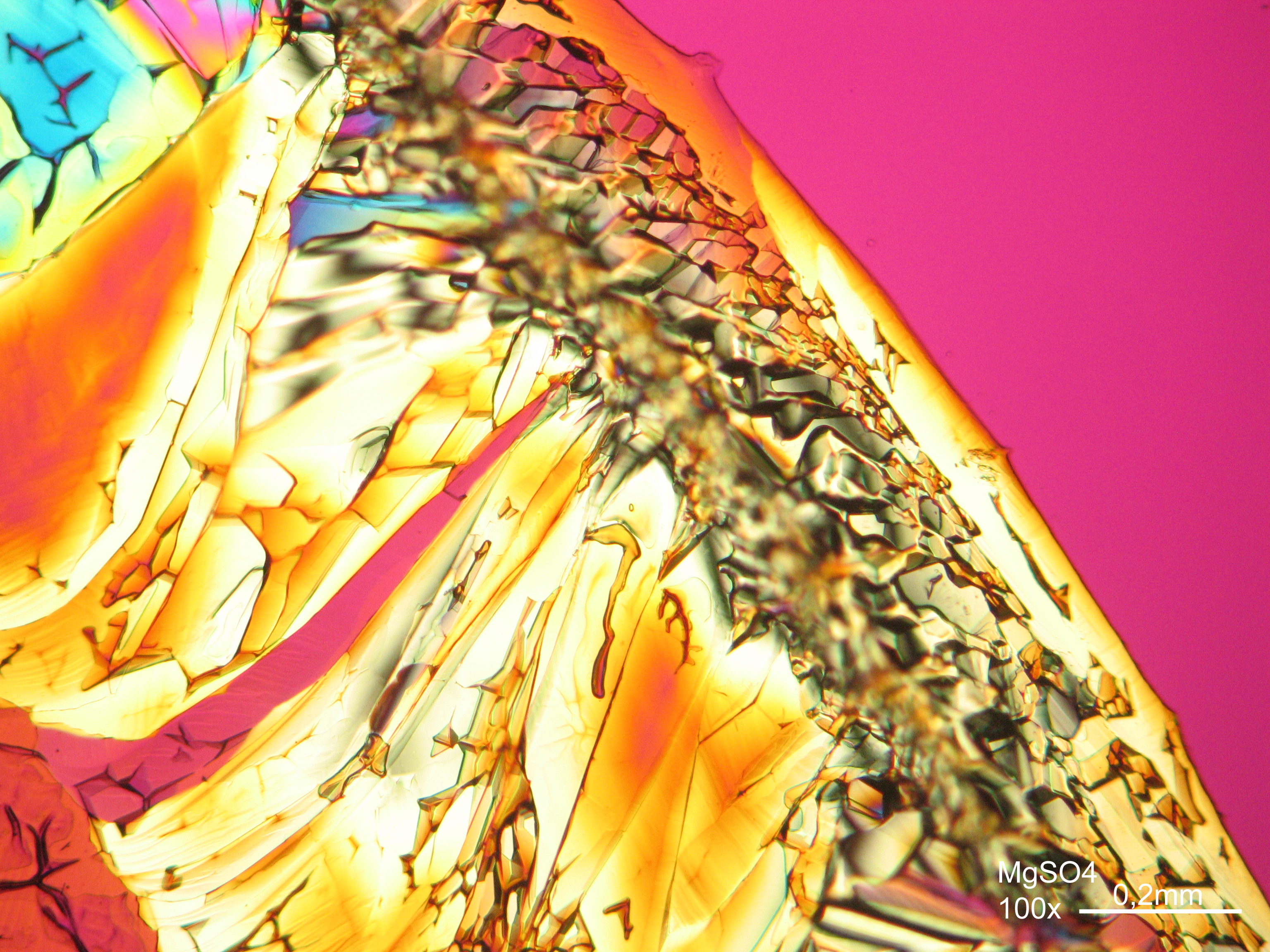
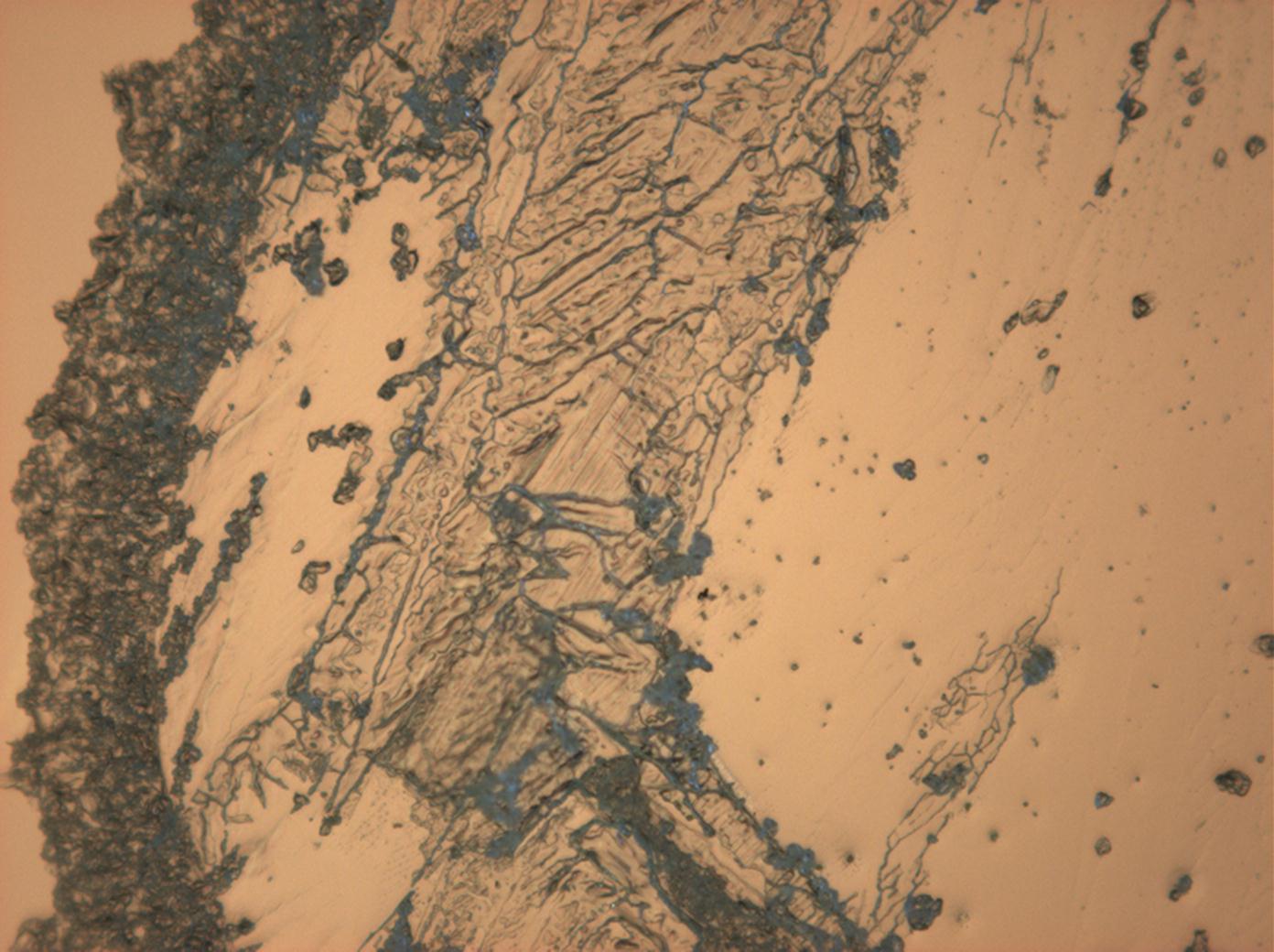
Magnesium sulfate[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Epsomite | MgSO4•7H2O | Δ = 0.0284 | nx = 1.432 ny = 1.453 nz = 1.4609 |
orthorhombic | biaxial negative |
- Magnesium sulfate crystallized from aqueous solution on a microscope slide
Sodium sulfate[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Thenardite | Na2SO4 | Δ = 0.015 | nx = 1.468 ny = 1.473 nz = 1.483 |
orthorhombic | positive |
- Sodium sulfate crystallized from aqueous solution on a microscope slide
Sodium carbonate[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Natrite | Na2CO3 | Δ = 0.131 | nx = 1.415 ny = 1.535 nz = 1.546 |
monoclinic | biaxial negative |
- Sodium carbonate crystallized from aqueous solution on a microscope slide
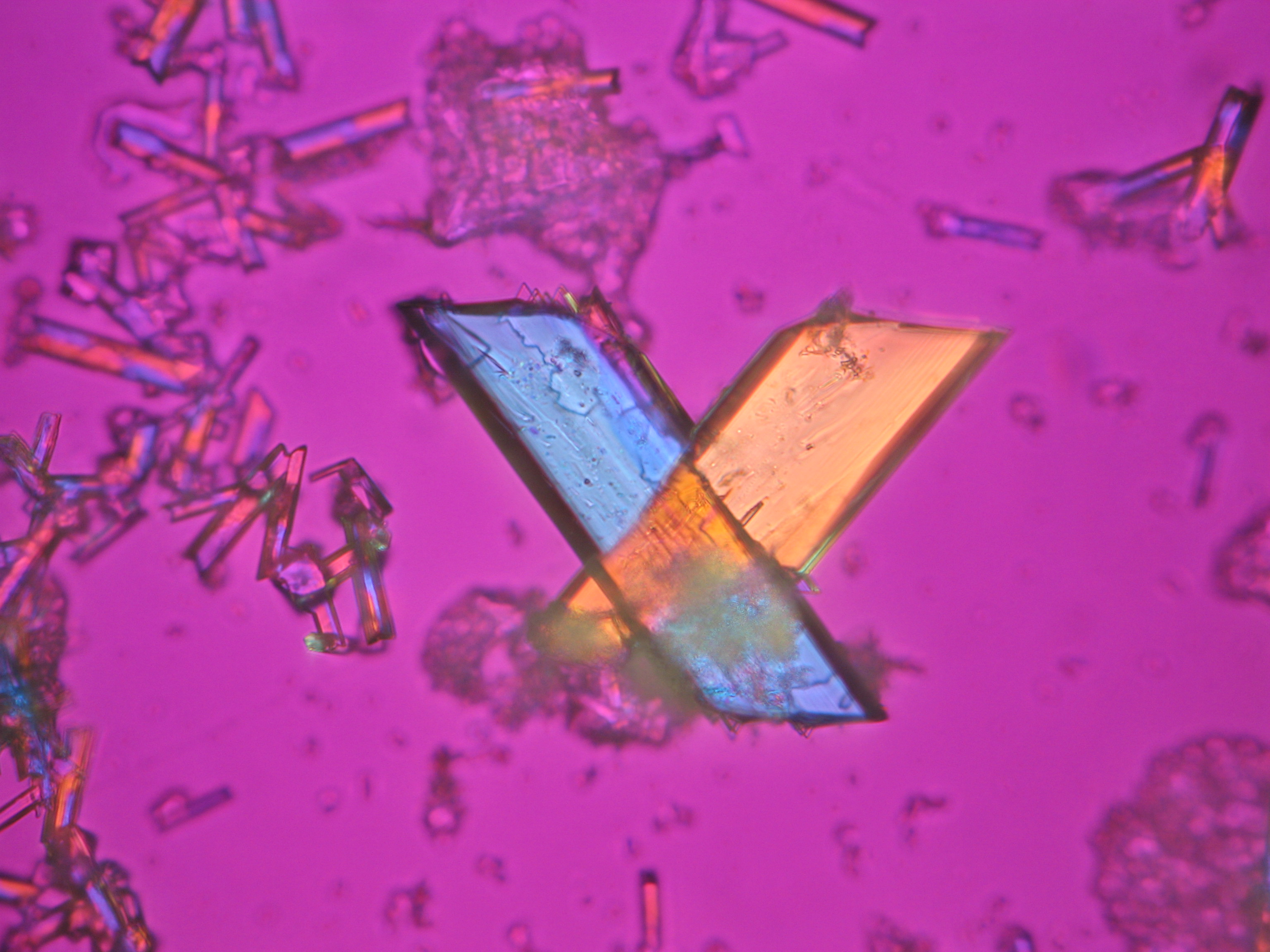
Sodium acetate[edit]
- Sodium acetate crystallized from aqueous solution
- Natriumacetat 2010 (1).JPG Figure 36: Sodium acetate crystals under polarized light, detail Figure 35
Weblinks[edit]
SLeithaeuser 13:33, 14 April 2012 (CEST)Titelvorschlag: Microscopic identification of salts