Sylvite: Difference between revisions
Jump to navigation
Jump to search
(Created page with "Category:Sylvite") |
No edit summary |
||
| (16 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Sylvite]] | {{Infobox_Salt | ||
|Footnote =<ref>http://webmineral.com/data/Sylvite.shtml viewed on 29/07/2010</ref><ref>http://www.mindat.org/min-3850.html viewed on 29/07/2010</ref> | |||
|photo = [[Image:HJS KCl-111703-03-10x.jpg|300px]] | |||
|mineralogical_Name =Sylvite, Hövelite | |||
|chemical_Name =Potassium chloride | |||
|Trivial_Name = | |||
|chemical_Formula =KCl | |||
|Hydratforms = | |||
|Crystal_System = cubic | |||
|Crystal_Structure = | |||
|Deliqueszenzhumidity =85.0% | |||
|Solubility = 4.595 mol/kg | |||
|Density =1.987 g/cm<sup>3</sup> | |||
|MolVolume =37.52 cm<sup>3</sup>/mol | |||
|Molweight =74.56 g/mol | |||
|Transparency =transparent to translucent | |||
|Cleavage =perfect | |||
|Crystal_Habit = | |||
|Twinning = | |||
|Refractive_Indices = n=1.4903 | |||
|Birefringence = | |||
|optical_Orientation =isotropic | |||
|Pleochroism = | |||
|Dispersion = | |||
|Phase_Transition = | |||
|chemBehavior = | |||
|Comments = | |||
|Literature = <bib id="Steiger.etal:2014"/> <bib id="Robie.etal:1978"/> <bib id="Dana:1951"/> | |||
}} | |||
back to [[Chloride]] | |||
==Solubility== | |||
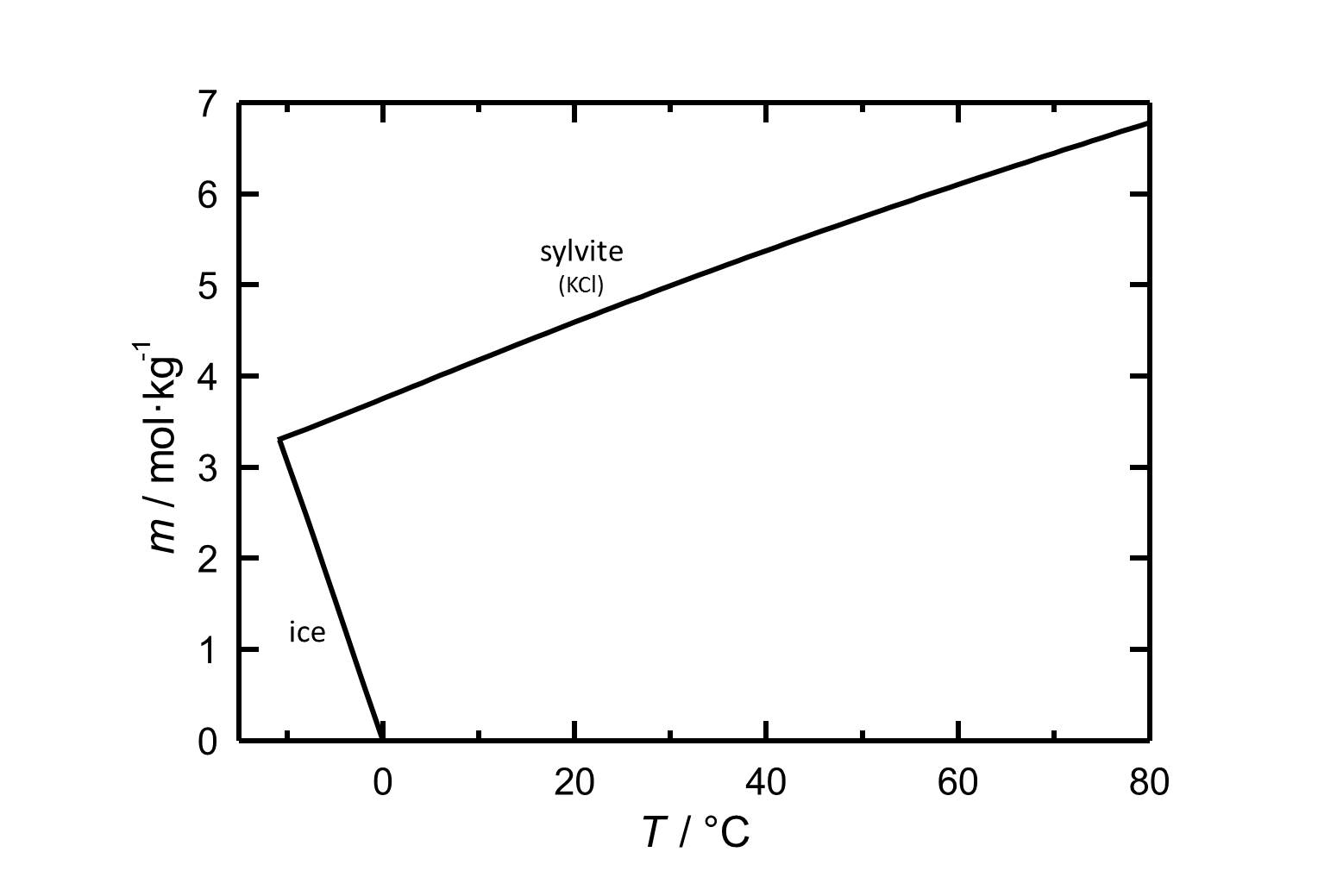
[[Image:S KCl e.jpg|800px|thumb|left|'''Figure 1''': Solubility of potassium chloride in water. The molality ''m'' [n(KCl)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted versus the temperature. According to <bib id="Steiger.etal:2008c"/>]] | |||
<br clear=all> | |||
==Hygroscopicity== | |||
<br clear=all> | |||
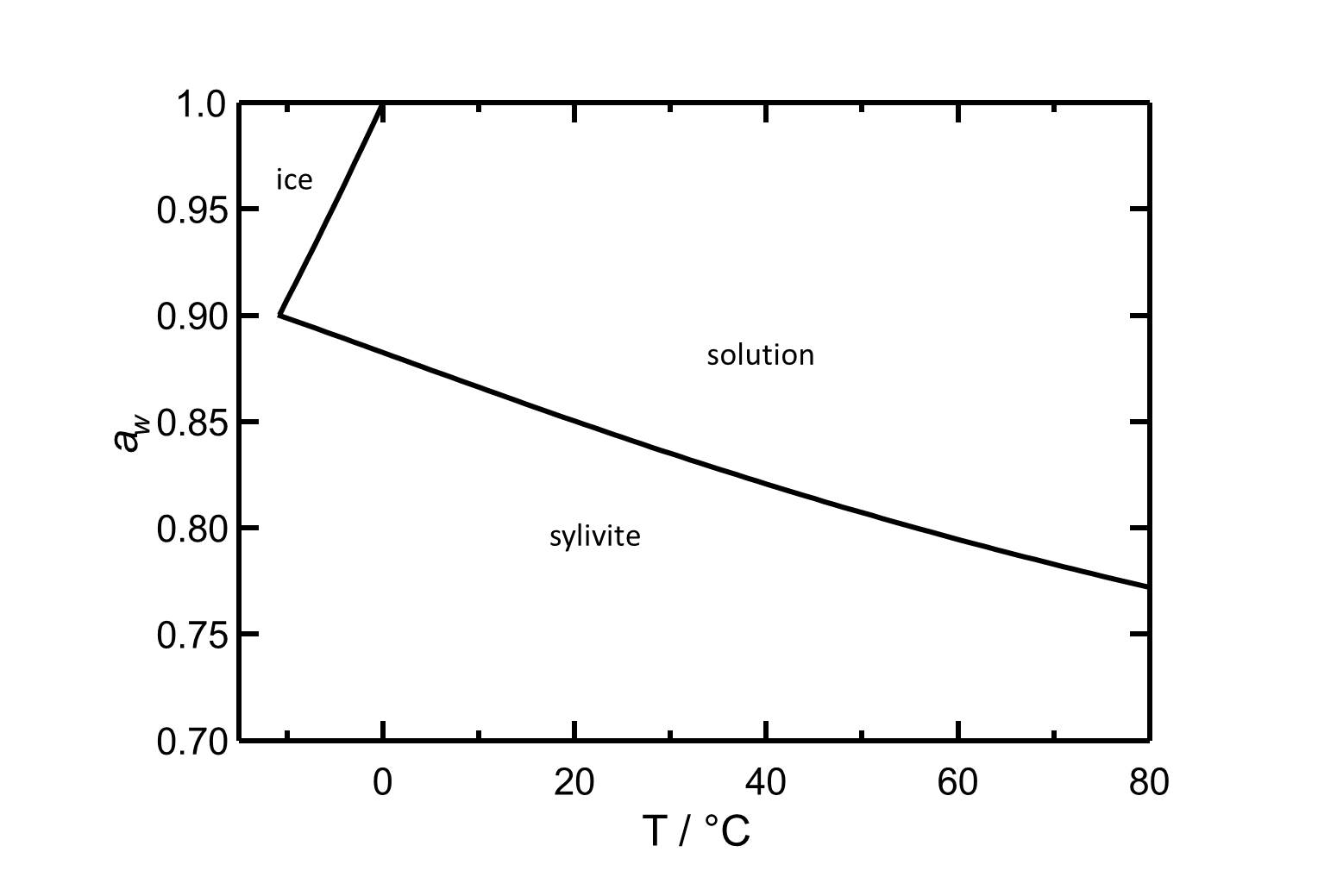
[[Image:D KCl e.jpg|800px|thumb|left|'''Figure 2''': Deliquescence behaviour of potassium chloride. The water activity ''a<sub>w</sub>'' is plotted versus the temperature. According to <bib id="Steiger.etal:2008c"/>]] | |||
<br clear=all> | |||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | |||
|+''Table 1: Deliquescence humidites of potassium chloride at different round temperatures <bib id="Steiger.etal:2014"/>'' | |||
|- | |||
|bgcolor = "#F0F0F0" align=center| 0°C | |||
|bgcolor = "#F0F0F0" align=center| 10°C | |||
|bgcolor = "#F0F0F0" align=center| 20°C | |||
|bgcolor = "#F0F0F0" align=center| 30°C | |||
|bgcolor = "#F0F0F0" align=center| 40°C | |||
|bgcolor = "#F0F0F0" align=center| 50°C | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| 88.3%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 86.7%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 85.0%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 83.5%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 82.1%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 80.7%r.h. | |||
|} | |||
<br clear=all> | |||
==Weblinks== | |||
<references/> | |||
== Literature== | |||
<biblist/> | |||
[[Category:Sylvite]][[Category:Chloride]][[Category:Salt]][[Category:InProgress]][[Category:List]] | |||
Latest revision as of 13:50, 17 March 2015
| Sylvite[1][2] | |

| |
| Mineralogical name | Sylvite, Hövelite |
| Chemical name | Potassium chloride |
| Trivial name | |
| Chemical formula | KCl |
| Other forms | |
| Crystal system | cubic |
| Crystal structure | |
| Deliquescence humidity 20°C | 85.0% |
| Solubility (g/l) at 20°C | 4.595 mol/kg |
| Density (g/cm³) | 1.987 g/cm3 |
| Molar volume | 37.52 cm3/mol |
| Molar weight | 74.56 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | n=1.4903 |
| Birefringence | |
| Optical Orientation | isotropic |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| [Steiger.etal:2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja  [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperaturesAuthor: Robie R.A., Hemingway B.S.; Fisher J.A.  [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D. 
| |
back to Chloride
Solubility[edit]

Figure 1: Solubility of potassium chloride in water. The molality m [n(KCl)•kg(H2O)-1] is plotted versus the temperature. According to [Steiger.etal:2008c]Title: An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code
Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

Hygroscopicity[edit]

Figure 2: Deliquescence behaviour of potassium chloride. The water activity aw is plotted versus the temperature. According to [Steiger.etal:2008c]Title: An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code
Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 88.3%r.h. | 86.7%r.h. | 85.0%r.h. | 83.5%r.h. | 82.1%r.h. | 80.7%r.h. |
Weblinks[edit]
- ↑ http://webmineral.com/data/Sylvite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-3850.html viewed on 29/07/2010
Literature[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger.etal:2008c] | Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas (2008): An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code. In: Construction and Building Materials, 22 (8), 1841-1850, 10.1016/j.conbuildmat.2007.04.020 |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |